
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
NSF/ANSI 61 – 2001
NSF International Standard/
American National Standard
Developed by a consortium of:
With support from:

NSF International, an independent, not-for-profit, non-governmental organization, is dedicated to being the leading global supplier of public health and safety-based risk management services serving the interests of all stakeholders.
This Standard is subject to revision.
Contact NSF to confirm this revision is current.
Users of this Standard may request clarifications and interpretations, or propose revisions by contacting:
Chair, Joint Committee on Drinking Water Additives
c/o NSF International
789 North Dixboro Road, P.O. Box 130140
Ann Arbor, Michigan 48113-0140 USA
Phone: (734) 769-8010 Telex: 753215 NSF INTL
FAX: (734) 769-0109 E-mail: info@nsf.org
Web: http://www.nsf.org
American National Standard/
NSF International Standard
for Drinking Water Additives—
Drinking water system components—
Health effects
Standard Developer
NSF International
Adopted February 9, 2001
NSF International Board of Directors
Designated as an ANSI Standard
February 9, 2001
American National Standard Institute
Prepared by
The NSF Joint Committee on Drinking Water Additives
Recommended for Adoption by
The NSF Council of Public Health Consultants
Adopted by
The NSF Board of Directors
June 1988
Revised October 1988
Revised May 1990
Revised May 1991
Revised May 1992
Revised September 1994
Revised January 1995
Revised July 1996
Revised September 1996
Revised November 1996
Revised January 1997
Revised March 1997
Revised July 1997
Revised November 1998
Revised January 1999
Revised November 1999
Revised September 2000
Revised November 2000
Revised February 2001
Published by
NSF International
PO Box 130140, Ann Arbor, Michigan 48113-0140, USA
For ordering copies or for making inquiries with regard to this Standard, please reference the designation “NSF/ANSI 61– 2001.”
Copyright 2001 NSF International
Previous editions © 2000, 1999, 1998, 1997, 1996, 1995, 1994, 1992, 1991, 1990, 1988
Unless otherwise specified, no part of this publication may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and microfilm, without permission in writing from NSF International.
Printed in the United States of America.
iiDisclaimers1
NSF, in performing its functions in accordance with its objectives, does not assume or undertake to discharge any responsibility of the manufacturer or any other party. The opinions and findings of NSF represent its professional judgment. NSF shall not be responsible to anyone for the use of or reliance upon this Standard by anyone. NSF shall not incur any obligation or liability for damages, including consequential damages, arising out of or in connection with the use, interpretation of, or reliance upon this Standard.
NSF Standards provide basic criteria to promote sanitation and protection of the public health. Provisions for mechanical and electrical safety have not been included in this Standard because governmental agencies or other national standards-setting organizations provide safety requirements.
Participation in NSF Standards development activities by regulatory agency representatives (federal, local, state) shall not constitute their agency's endorsement of NSF or any of its Standards.
Preference is given to the use of performance criteria measurable by examination or testing in NSF Standards development when such performance criteria may reasonably be used in lieu of design, materials, or construction criteria.
The illustrations, if provided, are intended to assist in understanding their adjacent standard requirements. However, the illustrations may not include all requirements for a specific product or unit, nor do they show the only method of fabricating such arrangements. Such partial drawings shall not be used to justify improper or incomplete design and construction.
Unless otherwise referenced, the annexes are not considered an integral part of NSF Standards. The annexes are provided as general guidelines to the manufacturer, regulatory agency, user, or certifying organization.
1 The information contained in this Disclaimer is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI’s requirements for an ANS. As such, this Disclaimer may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard.
iii iv| Foreword | ix | ||
| Consortium organizations | xi | ||
| 1 | Purpose, scope, and normative references | 1 | |
| 1.1 | Purpose | 1 | |
| 1.2 | Scope | 1 | |
| 1.3 | Normative references | 1 | |
| 1.4 | Limitations | 2 | |
| 1.5 | Alternate products or materials | 3 | |
| 2 | Definitions | 4 | |
| 3 | General requirements | 6 | |
| 3.1 | General | 6 | |
| 3.2 | Information and formulation requirements | 6 | |
| 3.3 | Identification of analytes of interest | 7 | |
| 3.4 | Products manufactured from annex C acceptable materials | 7 | |
| Table 3.1 – Material-specific analytes | 8 | ||
| 4 | Pipes and related products | 11 | |
| 4.1 | Scope | 11 | |
| 4.2 | Definitions | 11 | |
| 4.3 | General requirements | 11 | |
| 4.4 | Sample requirements | 12 | |
| 4.5 | Extraction procedures | 13 | |
| 4.6 | Analysis | 17 | |
| 4.7 | Normalization of contaminant concentrations | 17 | |
| 4.8 | Evaluation of contaminant concentrations | 19 | |
| Table 4.1 – Example single time point conditioning schedule | 20 | ||
| Table 4.2 – Single time point exposure schedule | 20 | ||
| Table 4.3 – Example multiple time point conditioning/exposure schedule | 21 | ||
| Table 4.4 – Pipes - normalization factors and assumptions | 22 | ||
| Table 4.5 – Fittings - (installed at regular intervals) normalization factors and assumptions | 23 | ||
| Table 4.6 – Example normalization calculations | 24 | ||
| 5 | Barrier materials | 25 | |
| 5.1 | Scope | 25 | |
| 5.2 | Definitions | 25 | |
| 5.3 | General requirements | 26 | |
| 5.4 | Sample requirements | 26 | |
| 5.5 | Extraction procedures | 27 | |
| 5.6 | Analysis of extraction water | 30 | |
| 5.7 | Normalization | 30 | |
| 5.8 | Evaluation of contaminant concentrations | 32 v | |
| Table 5.1 – Paint and coating system sample preparation | 33 | ||
| Table 5.2 – Single time point exposure sequence | 33 | ||
| Table 5.3 – Multiple time point exposure sequence | 34 | ||
| Table 5.4 – Surface area to volume ratios for tanks or storage vessels | 35 | ||
| 6 | Joining and sealing materials | 37 | |
| 6.1 | Coverage | 37 | |
| 6.2 | Definitions | 37 | |
| 6.3 | Material and extraction testing requirements | 37 | |
| 6.4 | Items of special significance | 37 | |
| 7 | Process media | 38 | |
| 7.1 | Scope | 38 | |
| 7.2 | Definitions | 38 | |
| 7.3 | General requirements | 38 | |
| 7.4 | Sample requirements | 39 | |
| 7.5 | Extraction procedures | 39 | |
| 7.6 | Analysis | 42 | |
| 7.7 | Normalization | 42 | |
| 7.8 | Evaluation of contaminant concentrations | 43 | |
| Table 7.1 – Product-specific minimum test batteries for process media products | 44 | ||
| Table 7.2 – Process media exposure dry weight per volume ratios | 45 | ||
| Table 7.3 – Maximum conditioning expansion rates for filtration and absorption media | 45 | ||
| Table 7.4 – Exposure schedule for process media of $0.25 mm in diameter | 46 | ||
| 8 | Mechanical devices | 47 | |
| 8.1 | Coverage | 47 | |
| 8.2 | Definitions | 47 | |
| 8.3 | Device, component, or material requirements | 47 | |
| Table 8.1 – Examples of mechanical devices | 49 | ||
| 9 | Mechanical plumbing devices | 50 | |
| 9.1 | Coverage | 50 | |
| 9.2 | Definitions | 51 | |
| 9.3 | Device, component, or material requirements | 51 | |
| 9.4 | Exposure and normalization | 52 | |
| 9.5 | Evaluation of normalized contaminant concentrations | 52 | |
| Annexes | |||
| A | Toxicology review and evaluation procedures (normative) | A1 | |
| A.1 | General requirements | A1 | |
| A.2 | Definitions | A1 | |
| A.3 | Data requirements for published risk assessments | A4 | |
| A.4 | Data requirements for new or updated risk assessments | A5 | |
| A.5 | Data requirements for evaluating short-term exposures | A6 | |
| A.6 | Risk estimation for published assessments | A7 | |
| A.7 | Risk estimation using new and updated risk assessments | A7 | |
| A.8 | Risk estimation for short-term exposure (STEL calculation) | A14 vi | |
| A.9 | Development of chemical class-based evaluation criteria | A15 | |
| A.10 | Key elements of a risk assessment for DWA chemicals | A16 | |
| References | A21 | ||
| Figure A1 – Annex A toxicity data review process | A22 | ||
| Figure A2 – Graphical presentation of data and extrapolations | A23 | ||
| Table A1 – Qualitative risk assessment data requirements | A24 | ||
| Table A2 – Quantitative risk assessment data requirements | A25 | ||
| Table A3 – TACs for qualitative risk assessment | A26 | ||
| Table A4 – Uncertainty factors | A27 | ||
| B | Product/material evaluation (normative) | B1 | |
| B.1 | Background | B1 | |
| B.2 | General evaluation requirements | B1 | |
| B.3 | Joining and sealing materials | B4 | |
| B.4 | Mechanical devices | B7 | |
| B.5 | Mechanical plumbing devices | B8 | |
| B.6 | Collection and preservation of extraction media following exposure | B10 | |
| B.7 | Analysis methods | B11 | |
| B.8 | Normalization | B17 | |
| B.9 | Extraction water preparation | B23 | |
| Figure B1 – Exposure sequence for mechanical plumbing devices | B25 | ||
| Table B1 – NSF/ANSI 61 products | B26 | ||
| Table B2 – Exposure summary | B26 | ||
| Table B3 – Extraction water selection | B27 | ||
| Table B4 – Test samples joining and sealing materials | B27 | ||
| Table B5 – Additional conditioning for hot application samples | B27 | ||
| Table B6 – Product exposure | B28 | ||
| Table B7 – Exposure sequence | B28 | ||
| Table B8 – Extractant water collection and preservation | B29 | ||
| Table B9 – Normalization factors, assumptions, and examples | B30 | ||
| Table B10 – Data available for determination of lead test statistic | B36 | ||
| Table B11 – Values of k1 for determining test statistic Q | B36 | ||
| Table B12 – Values of k2 for determining retest statistic R | B37 | ||
| Table B13 – One liter volume of extraction water | B37 | ||
| C | Acceptable materials (normative) | C1 | |
| C.1 | Background | C1 | |
| C.2 | Evaluation of acceptable materials | C1 | |
| C.3 | Extraction testing | C1 | |
| C.4 | Documentation | C1 | |
| Table C1 – Acceptable materials | C2 | ||
| D | Normative drinking water criteria (normative) | D1 | |
| D.1 | General | D1 | |
| D.2 | USEPA and Health Canada drinking water criteria | D1 | |
| D.3 | NSF International peer-reviewed drinking water criteria | D2 | |
| D.4 | Drinking water criteria based on EPA guidance concentrations | D2 | |
| D.5 | Threshold of evaluation (TOE) chemical list | D2 | |
| Table D1 – U.S. Environmental Protection Agency and Health Canada Standard 61 drinking water criteria | D3 vii | ||
| Table D2 – NSF International peer-reviewed drinking water criteria | D9 | ||
| Table D3 – Drinking water criteria based on USEPA guidance concentrations | D10 | ||
| Table D4 – Threshold of evaluation chemicals | D16 | ||
| E | Informational drinking water criteria (informative) | E1 | |
| E.1 | General | E1 | |
| E.2 | NSF International drinking water criteria (not externally peer-reviewed) | E1 | |
| E.3 | Informational threshold of evaluation chemicals | E2 | |
| Table E1 – NSF International drinking water criteria (not externally peer-reviewed) | E3 | ||
| Table E2 – Threshold of evaluation chemicals having datasets from which specific TAC/SPAC values could possibly be setting using Annex A | E11 | ||
In response to a competitive request for proposals from the U.S. Environmental Protection Agency (USEPA), a Consortium led by NSF International (NSF) agreed to develop voluntary third-party consensus standards and a certification program for all direct and indirect drinking water additives. Other members of the Consortium include the American Water Works Association Research Foundation, the Association of State Drinking Water Administrators, the Conference of State Health and Environmental Managers, and the American Water Works Association. (COSHEM has since become inactive as an organization.) Each organization was represented on a steering committee with oversight responsibility for the administration of the cooperative agreement. The Steering Committee provides guidance on overall administration and management of the cooperative agreement. Currently, the member organizations remain active in an oversight role.
Two standards for additives products were developed. NSF/ANSI 60 – Drinking water treatment chemicals—Health effects covers many of the water treatment chemicals, also known as direct additives. This Standard, NSF/ANSI 61 – Drinking water system components—Health effects, covers all indirect additives products and materials. Testing to determine the potential of a product to impart taste and/or odor to drinking water is not included in this Standard.
NSF/ANSI 61 was developed to establish minimum requirements for the control of potential adverse human health effects from products that contact drinking water. It does not attempt to include product performance requirements that are currently addressed in other voluntary consensus standards established by such organizations as the American Water Works Association, the American Society for Testing and Materials, and the American National Standards Institute. Because this Standard complements the performance standards of these organizations, it is recommended that products also meet the appropriate performance requirements specified in the standards of such organizations.
NSF/ANSI 61, and subsequent product certification against it, has replaced the USEPA Additives Advisory Program for drinking water system components. USEPA terminated its advisory role in April 1990. For more information with regard to USEPA’s actions, refer to the July 7, 1988 Federal Register (53FR25586).
This Standard and the accompanying text are intended for voluntary use by certifying organizations, utilities, regulatory agencies, and/or manufacturers as a basis of providing assurances that adequate health protection exists for covered products. Product certification issues, including frequency of testing and requirements for follow-up testing, evaluation, enforcement, and other policy issues, are not addressed by this Standard.
This Standard was initially approved by ANSI in 1989. NSF and all stakeholders have worked since 1988 to complete NSF/ANSI 61 by including requirements for mechanical plumbing products. Devices used within the final one L of the distribution system are covered under section 9 and include end-point devices such as faucets, glass fillers, water coolers, residential ice makers, and supply stops.
Section 9 was accepted by the NSF Joint Committee on Drinking Water Additives, and the NSF Council of Public Health Consultants. It was adopted by the NSF Board of Trustees on September 9, 1994, and approved by ANSI on September 12, 1995.
2 The information contained in this Foreword is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI’s requirements for an ANS. As such, this Foreword may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard.
ixWater contact materials in Drinking Water Treatment Units listed under NSF/ANSI 42, 44, 53, 55, 58, and 62 are tested and evaluated under a separate protocol from NSF/ANSI 61 with criteria which were developed specifically for the intended end-use. NSF 61 listing should not be additionally required for acceptance of these listed units for water contact application.
This version of the Standard (NSF/ANSI 61-2001) includes the following revisions:
– Minimum test batteries for common coating and paint materials were added to table 3.1.
– Section 4 was updated to specify that fitting products may be exposed as part of a pipe and fitting assembly to simulate their field exposure. For products having internal threaded areas, a requirement that 75% of the threaded area be covered during the exposure was added. Copper and copper alloy fittings can also be exposed using the pH 6.5 exposure water used for copper pipe. Normalization specifications for special use fittings were defined and an example normalization calculation provided.
– Section 5 requirements for Barrier Materials has been comprehensively updated. Technical revisions include the addition of guidelines for the determination of a representative test sample for similar product formulations, the exposure protocols were revised so that all sampling points are 24 hours in duration, the table for normalization factors was expanded to include many additional tank capacities, the assumptions for calculation of tank normalization factors were defined, and the sampling procedures for cement products was updated. The sample preparation, exposure, and normalization procedures have been moved from annex B to section 5.
At the suggestion of the NSF Council of Public Health Consultants, the terminology defining the drinking water criteria used in this Standard has been revised. The Maximum Drinking Water Level (MDWL) has been revised to the Total Allowable Concentration (TAC) and the Maximum Allowable Level (MAL) has been revised to the Single Product Allowable Concentration (SPAC).
This Standard was developed by the NSF Joint Committee on Drinking Water Additives using the consensus process described by the American National Standards Institute.
Suggestions for improvement of this Standard are welcome. Comments should be sent to Chair, Joint Committee on Drinking Water Additives, c/o NSF International, Standards Department, PO Box 130140, Ann Arbor, Michigan 48113-0140, USA.
xNSF International
Popularly referred to as NSF, NSF International is a noncommercial agency. It is incorporated under the laws of Michigan as a not-for-profit organization devoted to research, education, and service. It seeks to solve problems involving man and his environment. It wishes to promote health and enrich the quality of life through conserving and improving that environment. Its fundamental principle of operation is to serve as a neutral medium in which business and industry, official regulatory agencies, and the public come together to deal with problems involving products, equipment, procedures, and services related to health and the environment. It is conceived and administered as a public service organization.
NSF is perhaps best known for its role in developing standards and criteria for equipment, products, and services that bear upon health. NSF was the lead organization in the Consortium responsible for developing this Standard. NSF conducts research; tests and evaluates equipment, products, and services for compliance with standards and criteria; and grants and controls the use of NSF registered Marks.
NSF offers product certification (Listing Services) for all products covered by its standards. Each program has established policies governing the associated product evaluation, Listing Services, follow-up and enforcement activities. The NSF Listing Mark is widely recognized as a sign that the product or service to which it relates complies with the applicable NSF standard(s).
AWWA Research Foundation
The mission of the American Water Works Association Research Foundation (AWWARF) is to sponsor practical, applied research in behalf of the drinking water industry of North America. The scope of the research program embraces all aspects of water supply operation, from development and maintenance of water resources to treatment technologies and water quality issues, from storage and distribution system operations to health effects studies and utility planning and management activities. AWWARF serves as the centralized industry institution for planning, managing, and funding cooperative research and development in drinking water, including the subsequent transfer of technology and results for practical application by the water utility community.
AWWARF’s purpose in this cooperative program is to provide a communication link with the water utilities throughout North America and serve as the focal point for identification of research needs of the water supply industry with respect to the additives program.
The Association of State Drinking Water Administrators
The Association of State Drinking Water Administrators (ASDWA) is a nonprofit organization whose eligible membership is comprised of drinking water program administrators in each of the 50 states and seven U.S. territories. Through the organization, representatives speak with a collective voice to Congressional committees, the United States Environmental Protection Agency (EPA), professional and trade associations, water utilities, and the general public on issues related to state drinking water programs. With its mission of protecting the public health through assurance of high quality drinking water, and promoting responsible,
3 The information contained in this section is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI’s requirements for an ANS. As such, this section may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard.
xireasonable, and feasible drinking water programs at the state and federal levels, the Association is a valued contributor to the consortium, and to the program. It provides the link between the additives program and the state drinking water programs.
The Conference of State Health and Environmental Managers
The Conference of State Health and Environmental Managers (COSHEM), known formerly as the Conference of State Sanitary Engineers (CSSE), is currently inactive as an organization. It brought to the consortium expertise and involvement of state health and environmental program managers. The Conference was the focal point for health concerns of all state environmental programs, including drinking water, wastewater, air, solid and hazardous wastes, radiological, occupational, health, and food. A standing committee on water supply focused on drinking water issues and kept the membership informed. The Conference played an important role early in the program through two-way communication with state health and environmental program decision makers.
American Water Works Association
The purpose of the American Water Works Association (AWWA) is to promote public health, safety, and welfare by improving the quality and increasing the quantity of water delivered to the public, and to developing and furthering an understanding of the problems relating thereto by:
– advancing the knowledge of the design, construction, operation, water treatment and management of water utilities;
– developing standards for procedures, equipment, and materials used by public water supply systems;
– advancing the knowledge of problems involved in the development of resources, production, and distribution of safe and adequate water supplies;
– educating the public on the problems of water supply and promoting a spirit of cooperation between consumers and suppliers in solving these problems; and
– conducting research to determine the causes of problems of providing a safe and adequate water supply and proposing solutions thereto in an effort to improve the quality and quantity of the water supply provided to the public.
AWWA brings to the Consortium its established position as the largest public drinking water association in North America, with a broad membership that includes utilities, consultants, manufacturers/distributors/agents, contractors, and other organizations with a direct interest in drinking water.
xiiNSF/ANSI Standard for Drinking Water Additives—
Drinking water system components—Health effects
1.1 Purpose
This Standard establishes minimum health effects requirements for the chemical contaminants and impurities that are indirectly imparted to drinking water from products, components, and materials used in drinking water systems. This Standard does not establish performance, taste and odor, or microbial growth support requirements for drinking water system products, components, or materials.
1.2 Scope
1.2.1 This Standard is intended to cover specific materials or products that come into contact with: drinking water, drinking water treatment chemicals, or both. The focus of the standard is evaluation of contaminants or impurities imparted indirectly to drinking water. The products and materials covered include, but are not limited to, process media (carbon, sand, etc.), protective materials (coatings, linings, liners, etc.), joining and sealing materials (solvent cements, welding materials, gaskets, etc.), pipes and related products (pipes, tanks, fittings, etc.), mechanical devices used in treatment/transmission/distribution systems (valves, chlorinators, separation membranes, etc.), and mechanical plumbing devices (faucets, endpoint control valves, etc.).
1.2.2 Point-of-use and point-of-entry drinking water treatment devices are not covered by the scope of this Standard.
1.2.3 Fire hydrants are not covered by the scope of this Standard.
1.3 Normative references
The following documents contain requirements which, by reference in this text, constitute requirements of this Standard.
APHA, Standard Methods for the Examination of Water and Wastewater, twentieth edition1)
ASTM C31/C31M-96, Standard Practice for Making and Curing Concrete Test Specimens in the Field2)
ASTM C109/C109M-98, Standard Test Method for Compressive Strength of Hydraulic Cement Mortars2)
ASTM C192/C192M-95, Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory2
1) American Public Health Association (APHA), 800 I Street, NW, Washington, DC 20001
2) American Society for Testing and Materials (ASTM), 100 Barr Harbor Drive, West Conshohocken, PA 19428-2859
1ASTM C511-97, Standard Specification for Moist Cabinets, Moist Rooms, and Water Storage Tanks Used in the Testing of Hydraulic Cements and Concretes2)
ASTM C778-97, Standard Specification for Standard Sand2)
ASTM D1193-99, Standard Specification for Reagent Water2)
ASTM D2855-96, Standard Practice for Making Solvent-Cemented Joints with Poly(Vinyl Chloride) (PVC) Pipe and Fittings2)
ASTM D3182-89, Standard Practice for Rubber - Materials, Equipment, and Procedures for Mixing Standard Compounds and Preparing Standard Vulcanized Sheets2)
ASTM F493-97, Standard Specification for Solvent Cements for Chlorinated Poly(Vinyl Chloride) (CPVC) Plastic Pipe and Fittings2)
ANSI/AWWA B100-96, AWWA Standard for Filtering Material3)
ANSI/AWWA C652-92, AWWA Standard for Disinfection of Water-Storage Facilities3)
NSF/ANSI 60 - 2001, Drinking Water Treatment Chemicals - Health Effects
OECD, OECD Guidelines for the Testing of Chemicals, May 19964)
USEPA-600/4-79-020, Methods for the Chemical Analysis of Water and Wastes, March 19835)
USEPA-600/4-80-032, Prescribed Procedures for Measurement of Radioactivity in Drinking Water5)
USEPA Health Effects Testing Guidelines, 40 CFR Part 79866)
USEPA Good Laboratory Practice Standards, 40 CFR Part 7926)
USEPA Good Laboratory Practice for Non-Clinical Laboratory Studies, 21 CFR 587)
USFDA, Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives in Food7)
1.4 Limitations
The requirements of this Standard are limited to addressing potential health effects, except where specific application and performance standards are referenced. This Standard does not establish taste and odor requirements for drinking water system products and materials. The criteria set forth in this Standard cover products produced by good manufacturing practices and generally recognized manufacturing processes. As
3) American Water Works Association (AWWA), 6666 Quincy Avenue, Denver, CO 80235-9913
4) Organization for Economic Cooperation and Development (OECD), 2 Rue Andre-Pascal, 75775 Paris Cedex 16, France
5) USEPA, Environmental Monitoring and Support Laboratory, Cincinnati, OH 45268
6) Superintendent of Documents, U.S. Government Printing Office, Washington, DC 20402
7) USFDA, 5600 Fishers Lane, Rockville, MD 20857
2the presence of unusual or unexpected impurities is frequently dependent upon the method of manufacture and the quality of raw material used, products prepared by other than recognized methods of manufacture or with unusual raw materials shall be fully evaluated in accordance with section 3 of this Standard (general requirements). Products that have been evaluated and found to meet other NSF standards having health requirements equivalent to this Standard as indicated in each section shall be acceptable for drinking water applications without separate evaluation under this Standard8)
1.5 Alternate products or materials
While specific materials are stipulated in this Standard, drinking water system products or components that incorporate alternate materials shall be acceptable when it is verified that the product or component meets the applicable requirements of the Standard based on its end use.
8) Final acceptance of a product for drinking water application is the responsibility of the appropriate federal, state, or local regulatory agent.
3Terms used in this Standard that have a specific technical meaning are defined here.
2.1 analytical summary: A list of the analytes and analytical procedures, both chemical and microbiological, which are selected to determine whether a product is compliant to the requirements of the Standard; analytes may be either product specific or formulation dependent.
2.2 at the tap: Referring to the point-of-delivery or point-of-use for drinking water.
2.3 cold water application: A product application which is not intended to result in exposure for extended periods to water in excess of ambient water temperature.
2.4 contaminant: Any physical, chemical, biological, or radiological substance or matter in water.
NOTE – Consistent with the definition in the Federal Safe Drinking Water Act, a contaminant can have either a beneficial or detrimental effect on the potability of water.
2.5 direct additives: A treatment chemical and its contaminants directly added to water during the production of drinking water.
2.6 distribution system: The system of conduits or the network of pipelines (located primarily in the streets) through which a primary domestic water supply is distributed to consumers. In plumbing codes this term is applied to all the hot and cold water piping installed in buildings.
2.7 drinking water: Water intended for human consumption.
2.8 good manufacturing practices: The practice of maximizing the purity of products and materials by maintaining and practicing appropriate quality control and quality assurance procedures.
2.9 hot water application: A product application which is intended to result in exposure for extended periods to water which has been raised from ambient temperature.
2.10 indirect additives: Contaminants which are extracted into drinking water through contact with the surfaces of materials, components, or products used for its treatment, storage, transmission, or distribution.
2.11 manufacturer: A corporation, company, or individual that produces, formulates, packages, or repackages products, components, and materials that are intended to be in contact with drinking water.
2.12 maximum contaminant level (MCL): The maximum concentration of a regulated contaminant that is permitted in a public drinking water supply, as defined under the Federal Safe Drinking Water Act.
NOTE – If the manufacturer requests review to relevant alternate regulatory requirements, the certifying agency can consider alternative regulatory levels, e.g. Canadian Maximum Acceptable Concentrations (MACs).
2.13 normalization: The process of adjusting laboratory extraction results by accounting for differences between laboratory and field surface area-to-volume ratios to reflect the contaminant concentration at the tap.
2.14 normalized concentration: A value for a contaminant concentration from a laboratory extraction test which has been adjusted to reflect the potential contaminant concentration at the tap.
2.15 point-of-entry system: A system with an inlet connection of 25.4 mm (1 in) or less which contacts all or a majority of the water entering the facility.
2.16 point-of-use system: A system located at a single tap or multiple taps which does not contact the
4majority of water entering the building or residence.
2.17 short-term exposure level (STEL): A maximum concentration of a contaminant which is permitted in drinking water for an acute exposure calculated in accordance with annex A of this Standard.
2.18 single product allowable concentration (SPAC): The maximum concentration of a contaminant in drinking water that a single product is allowed to contribute as defined by annex A of this Standard.
2.19 total allowable concentration (TAC): The maximum concentration of a nonregulated contaminant allowed in a public drinking water supply as defined by annex A of this Standard.
2.20 transmission system: A system of conduits through which a primary water supply is transmitted to the distribution system.
53.1 General
3.1.1 Product and material information described in 3.2 shall be used to determine the specific section (sections 4 through 9) under which a product or material shall be evaluated.
3.1.2 Products or materials whose intended uses fall under more than one section of this Standard shall be evaluated under the section having the most rigorous evaluation conditions.
NOTE – Rigorous conditions are typically associated with shorter conditioning periods, longer exposure periods, higher surface area-to-volume ratios, and higher exposure temperatures.
3.2 Information and formulation requirements
The following information shall be reviewed to determine the appropriate analytical testing and to ensure that the potential health effects of products or materials are accurately and adequately identified:
6– the product section(s) under which the product, component, or material is covered, and the intended function or end use of the product or the material;
– for assembled products or components, a list of all of components and materials and their corresponding surface areas which come into direct contact with water;
– when appropriate, the total volume of water that the product can hold when filled to capacity;
– the expected service life of the product;
– the anticipated minimum, maximum, and average volumes of water that come into contact with the product, component, or material during a 24-hour period;
– complete formulation information for each water contact material as applicable;
– the composition of the formulation (e.g. percent or parts by weight for each chemical in the formulation or reference to a standardized material specification);
– a chemical abstract number (CAS no.), name, trade designation, and supplier for each chemical present in the formulation, and a Material Safety Data Sheet (MSDS) when available;
– an indication as to whether the chemical is an ingredient, reactant, or processing aid.
– the maximum temperature to which the product, component, or material is exposed during its intended end use;
– a description/classification of the manner in which the product or material is manufactured, handled, and packaged;
– when available, a list of the known or suspected impurities within the product or material, and the maximum percent or parts by weight of each impurity;
– when available, the solubility, hydrolysis products, and extraction rates of chemicals within the product or material; and
– when available, a list of published and unpublished toxicological studies relevant to the chemicals and impurities present in the product, component, or material.
3.3 Identification of analytes
For all products and materials, the formulation information required in 3.2 shall be reviewed for completeness (e.g., all formulations total 100%), and to determine whether a minimum test battery has been established for each water contact material (see table 3.1). The availability of an established minimum test battery shall not preclude performance of a formulation review to identify any formulation-dependent analytes (see 3.3.1).
3.3.1 Formulation-dependent analysis selection
For all water contact materials, the formulation information described in section 3.2 shall be reviewed and formulation-dependent analytes shall be identified for each water contact material. The criteria for selection of a formulation dependent analyte shall include, but not be limited to the following:
– known or suspected toxicity of the substance or its byproduct(s);
– high water solubility of the substance;
– monomer(s) of polymeric ingredients;
– high probability of extraction of a substance or its byproduct(s) at toxicologically significant concentrations; and
– extraction or migration information for the substance provided by the manufacturer.
3.3.2 Established minimum test batteries
The materials listed in table 3.1 shall be tested for the indicated analyses and any formulation-dependent analyses identified during the formulation-dependent analyte selection.
3.4 Products manufactured from annex C acceptable materials
Products manufactured entirely from annex C materials shall not be required to undergo extraction testing for material-specific analytes of interest. However, extraction testing for contaminants contributed by processes specific to a production site shall be considered as formulation-dependent analytes. Annex C contains the evaluation requirements for qualification as an acceptable material.
7| Material type | Required analyses |
|---|---|
| Pipe/fitting/device materials | |
| Asphaltic-coated ductile iron | GC/MS base/neutral scan (specific for carbonyls and non-aromatic hydrocarbons)1), volatile organic chemicals (VOCs), polynuclear aromatic hydrocarbons (PNAs), regulated metals2), molybdenum, vanadium, manganese |
| Brass | regulated metals2), zinc, nickel |
| Concrete | regulated metals2) |
| Copper | regulated metals2) |
| Galvanized steel | regulated metals2), zinc, nickel |
| Stainless steel | regulated metals2), nickel |
| Plastic materials | |
| Acetal (AC)/Polyoxymethylene (POM) | formaldehyde, VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1), acetal oligomers (by GC/MS base/acid scan)1) |
| Acrylonitrile-butadiene-styrene (ABS) | acrylonitrile, 1,3-butadiene, styrene, regulated metals2), VOCs, phenolics (by GC/MS based/acid scan)1) |
| Cross linked polyethylene (PEX) | GC/MS1), VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1), methanol, tert-butyl alcohol3) |
| Nylon 6 | caprolactam, nitrogen-containing extractants (by GC/MS base/neutral scan)1), VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Other nylons | nitrogen-containing extractants (by GC/MS base/neutral scan)1), VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1), nylon monomers |
| Polybutylene (PB) | VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polyethylene (PE) | VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polyphenylene oxide (PPO) | dimethyl phenol, VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polyphthalamide (PPA) | hexamethylene diamine, terephthalic acid, isophthalic acid, VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polypropylene (PP) | VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polysulphone including poly[phenylene sulphone] (PPSU) | sulphone monomer, VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polysulphone including poly [phenylene sulphone] (PPSU) | sulphone monomer, VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polyurethane (PUR) | GC/MS1), VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1) |
| Polyvinyl chloride (PVC) and Chlorinated polyvinyl chloride (CPVC) | regulated metals2), phenolics1), VOCs, tin4), antimony5), residual vinyl chloride monomer (RVCM)6) |
| Polyvinyl chloride (flexible) | VOCs, regulated metals2), phenolics (by GC/MS base/acid scan)1), phthalates7), RVCM6), tin4), zinc8) 8 |
| Elastomer materials | |
| Ethylene-propylene-diene monomer (EPDM) | GC/MS1), VOCs, phenolics (by GC/MS base/acid scan)1), phthalates7), PNAs1) |
| Fluoroelastomer | GC/MS1), VOCs, phthalates7), |
| Isoprene | GC/MS1), VOCs, phenolics (by GC/MS base/acid scan)1), phthalates7), PNAs1), isoprene monomer |
| Neoprene | GC/MS1), VOCs, phenolics (by GC/MS base/acid scan)1), phthalates7), PNAs1), chloroprene |
| Nitrile-butadiene rubber (NBR, BUNA-N) | GC/MS1). VOCs, phenolics(by GC/MS base/acid scan)1), phthalates7), PNAs1), 1,3-butadience, acrylonitrille |
| Styrene-butadiene rubber (SBR) | GC/MS1), VOCs, phenolics (by GC/MS base/acid scan)1), phthalates1), PNAs1), 1,3-butadience, styrene |
| Barrier materials | |
| Asphaltic coatings | regulated metals2), molybdenum, vanadium, manganese, VOCs, GC/MS base/neutral scan (specific for carbonyls an non-aromatic hydrocarbons)1), PNAs1) |
| Epoxy coatings (liquid and powder) | GC/MS (base/neutral/acid scan), bisphenol A, bisphenol A-diglycideryl ether9), bisphenol A-diglycideryl ether9), bisphenol A-propoxylate9), epichlorohydrin, VOCs, solvent and reactive diluents additives10) |
| Polyester coatings | GC/MS (base/neutral/acid scan), VOCs, residual monomers11) |
| Polyester coatings | GC/MS (base/neutral/acid scan), VOCs |
| Portland and hydraulic cements | GC/MS1), regulated metals2), dioxins and furans12), radionuclides, glycols and ethanolamines13) 9 |
| 1) See annex B, section B.7 2) Antimony, arsenic, barium, beryllium, cadmium, chromium, copper, lead, mercury, selenium, thallium 3) tert-butyl alcohol analysis is required for PEX materials except those crosslinked via 3-beam methodology. 4) The analysis for tin is required when tin-based stabilizers are used. 5) The analysis for antimony is required when antimony-based stabilizers are used. 6) The level of RVCM within the walls of PVC or CPVC products and materials shall be directly determined (annex B, section B,7). 7) The analysis for phthalates is required when phthalate ester plasticizers are used. Analysis shall be for the specific phthalate ester(s) used in the formulation. 8) The analysis for zinc is required when zinc-based stabilizers are used. 9) Analysis shall be performed using Liquid Chromatography with Ultraviolet detection (LC/UV). 10) Analysis shall be performed for the specific solvent and reactive diluents additives used in the individual product formulation, such as benzyl alcohol. 11) Analysis shall be performed for residual concentrations of the specific ester monomer’s used in the individual product formulation. 12) Dioxin and furan analysis to be performed on cements using fuel or material sources which are defined as hazardous waste by the U.S. Resource Conservation and Recovery Act (RCRA). 13) Glycol and ethanolamine analyses shall be performed on cements containing these compounds as grinding aids. |
|
4.1 Scope
4.1.1 The requirements in this section apply to pipes and pipe-related products and the water-contact materials associated with these products. Pipe-related products include, but are not limited to, the following items: fittings, couplings, flexible and rigid tubing, riser tubing, dip tubes, hoses, well casings, drop pipes, screens, and pipe-related coatings.
4.1.2 Coatings and other barrier materials not exclusively intended for application to pipes or pipe-related products are evaluated under 5.
NOTE – Coatings and other barrier materials which meet the requirements of 5 at a specific surface area-to-volume ratio, shall be considered to meet the requirements of a pipe or pipe-related product application for a surface area-to-volume ratio less than or equal to the ratio accepted under the 5 evaluation.
4.1.3 Individual ingredients of cement-based pipes and related products (including portland and blended hydraulic cement and admixtures) are evaluated under 5.
4.1.4 Products and materials intended to join or seal pipes or pipe-related products are evaluated under 6.
4.2 Definitions
4.2.1 Cold water application: A product application which is intended to result in continuous exposure to water of ambient temperature. Products are tested for an end-use temperature of 23 ± 2EC (73 ± 4EF).
4.2.2 commercial hot water applications: A product application which is intended to result in continuous or intermittent exposure to water which has been raised from ambient temperature. Intermittent exposure is considered to be any hot water contact which is not continuous. Products are tested for an end-use temperature of 82 ± 2EC (180 ± 3EF).
4.2.3 domestic hot water applications: A product application which is intended to result in continuous or intermittent exposure to water which has been raised from ambient temperature. Intermittent exposure is considered to be any hot water contact which is not continuous. Products are tested for an end-use temperature of 60 ± 2EC (140 ± 3EF).
4.2.4 nominal diameter: A designation system used to specify a pipe size, where the designation for a specific size is approximately equal to the average inside diameter of the pipe.
4.3 General requirements
4.3.1 The product size with the most conservative normalization conditions shall be evaluated. Successful evaluation of such product shall qualify all products of less conservative normalization conditions, provided the materials of construction are identical as specified in 4.4.1.
11NOTE – For products of ½ in to 4 in nominal diameter (1.3 cm to 10 cm) and products of 4 in diameter and greater (10 cm and greater), the most stringent normalization condition is typically the smallest inner diameter product within the nominal diameter range. Products of less than ½ in nominal diameter (1.3 cm) are assumed to have limited exposure in the distribution system (see assumptions in tables 4.4 and 4.5). Successful qualification of products of less than ½ in nominal diameter (1.3 cm) may not demonstrate the acceptability of all products ½ in nominal diameter (1.3 cm) and greater.
4.3.2 Residual vinyl chloride evaluation
Polyvinyl chloride and chlorinated polyvinyl chloride products and materials shall be evaluated for the level of residual vinyl chloride monomer (RVCM) in the product wall or in the material according to annex B, section B.7.
4.4 Sample requirements
4.4.1 General
A sample can represent a product line of various sizes when:
– materials are of the same alloy, composition, or formulation;
– materials have undergone the same manufacturing process, e.g., casting, extrusion;
– designs and manufacturing processes are analogous; and
– it has the most stringent normalization requirements (see 4.3.1).
4.4.2 Materials
When a material is proposed for evaluation, a representative sample of the material shall be used. Material test samples (e.g. plaque or sheet) shall be used only if no chemical or physical difference exists between the material sample and the material as it is used in applications covered by 4. A material intended to be processed by more than one method (e.g. injection molding, extrusion, stamping, etc.) shall be tested in each of its processed forms.
4.4.3 Finished products
When a finished product (e.g. pipe or fitting) is proposed for evaluation, a sample of the finished product shall be used of testing except in the following specific instances:
– concrete cylinders, cubes, or other concrete surrogate samples can be evaluated on behalf of concrete-lined pipes and other concrete-based products;
– coatings, applied to the appropriate substrate, can be evaluated on behalf of products whose entire water contact surface is covered by the coating; and
– Finished products, for which finished product evaluation is impractical due to one or more of the following reasons, shall be permitted to be evaluated using material samples:
a) an internal volume greater than 20 L (5.3 gallons);
b) a weight greater than 34 kilograms (75 lbs);
c) in situ manufacture of the finished product.
Material samples shall be permitted to be evaluated on behalf of a finished product if the first and second criteria listed under 4.4.1 are satisfied.
124.5 Extraction procedures
4.5.1 Analytical summary
An analytical summary shall be prepared for each product or material. The analytical summary shall consist of the formulation-dependent analytes identified in 3.2 and the applicable material-specific analytes listed in table 3.1.
4.5.2 Preparation of test samples
4.5.2.1 In all cases, test samples shall be prepared so that the laboratory surface area-to-volume ratio is equal to or greater than the surface area-to-volume ratio at which the product is intended to be used in the field.
NOTE – To facilitate the exposure of product samples that are connected to pipe or tubing products under normal installation conditions (e.g., fittings), the samples may be attached to lengths of pipe or tubing of the appropriate nominal diameter. When preparing a test sample in this manner, the exposed surface area of the fitting test sample shall represent a percentage of the total exposed surface area (test sample plus the attached pipe or tubing) that is equal to or greater than the percentage specified in the table 4.5 normalization assumptions, for the specific nominal diameter and end use of the product (flexible or rigid piping system). The pipe or tubing material shall also be present in the method blank as required in annex B, section B.2.8.1.
4.5.2.2 Unless manufacturer’s instructions direct otherwise, test samples shall be rinsed in cold tap water until any extraneous debris or contamination which occurred during shipping and handling is removed. The samples shall then be rinsed in deionized water (ASTM D 1193 Type II).
4.5.2.3 If the exterior surface of a product is to be exposed, all markings which are not integral to the product (e.g. ink markings) shall be removed.
4.5.2.4 When the test sample contains internal threaded outlets, 75% of the threaded surface area shall be covered by insertion of a threaded component of the appropriate diameter to produce a water tight seal.
4.5.3 Exposure water
4.5.3.1 General
Exposure water selection shall be determined by the analytes of interest identified on the analytical summary (see 4.5.1). Exposure water(s) shall be selected in accordance with annex B, section B.2.5.
4.5.3.2 Copper and copper alloys
Copper and copper alloy pipe and tubing shall be exposed in the pH 6.5 and in the pH 10 exposure waters as described in annex B, section B.9. Copper and copper alloy fittings intended to be used with copper pipe and tubing shall be exposed in either the pH 5 or the pH 6.5 exposure waters (at the discretion of the manufacturer) and in the pH 10 exposure water, as described in annex B, section B.9. For all copper and copper alloy pipes, tubing, and fittings tested using the pH 6.5 exposure water, the manufacturer’s literature shall indicate this use limitation. The manufacturer’s use instructions or product literature that references this Standard shall indicate this use limitation by inclusion of the following statement.
“Copper [tube, pipe, or fitting] (Alloy [alloy designation]) has been evaluated by [Testing Organization] to NSF/ANSI 61 for use in drinking water supplies of pH 6.5 and above. Drinking water supplies which are less than pH 6.5 may require corrosion control to limit leaching of copper into the drinking water.”
134.5.4 Conditioning and exposure options
4.5.4.1 In-product conditioning and exposure
During in-product conditioning and exposure, the test sample shall be filled completely with exposure water. The product having the greatest surface area-to-volume ratio (typically the smallest diameter) shall be preferentially used. When necessary to prevent the loss of exposure water, samples shall be capped with inert materials (e.g. glass).
4.5.4.2 In-vessel conditioning and exposure
During in-vessel conditioning and exposure, samples shall be placed in containers composed of a material that is inert to the exposure water having polytetrafluoroethylene lined lids. The exposure water shall completely immerse the sample. All samples shall be exposed at a surface area-to-volume ratio which is equal to or greater than that of the intended end use. The actual wetted surface area-to-volume ratio achieved during the exposure shall be recorded.
NOTE – The stated duration of the conditioning period at the hot temperature does not include any time needed to elevate the product sample or exposure vessel to the required exposure temperature.
4.5.4.3 Multiple time point protocol
When the normalized concentration of a contaminant exceeds, or is expected to exceed, its acceptable level when evaluated as a single time point exposure (see 4.5.6), determination of the contaminant leaching rate as a multiple time point exposure shall be considered (see 4.5.7). For the purpose of contaminant concentration evaluation (see 4.8.2), Day 1 shall be defined as the time point at which extractant water is collected for analysis under the single time point exposure protocol in table 4.2 (17 days in elapsed time). Day 90 shall be defined as 90 days following this time point.
NOTE – When employing a multiple time point protocol to the evaluation of a contaminant, consideration shall be given to the leaching characteristics of the contaminant, e.g. does the leaching pattern demonstrate a linear regression. Consideration shall also be given to the availability of appropriate toxicity data to define an acute exposure limit for the contaminant, as required in 4.8.2.
4.5.5 Single time point conditioning protocols
A separate sample shall be conditioned for each type of exposure water selected in 4.5.3.
4.5.5.1 Single time point conditioning –cold application
Products which are intended to be in contact with only cold water shall be conditioned in the exposure water(s) selected in 4.5.3 at 23 ±;2°C (73 ± 4°F) for 14 days. During the 14-day period, the exposure water shall be changed at least 10 times with a minimum period of 24 ± 1 hour between water changes. The free available chlorine concentration during the conditioning period shall be 2 mg/L. After the 14-day conditioning period, the exposure water in the product or in the vessel shall be decanted and discarded. Shortened conditioning periods shall be used at the request of the manufacturer. Exposure of the sample according to 4.5.6 shall immediately follow conditioning.
14NOTE – Table 4.1 provides an example single time point conditioning protocol. Alternate protocols shall be permitted as long as the requirements of 4.5.5.1 are met.
4.5.5.2 Single time point conditioning–hot applications
4.5.5.2.1 Intermittent hot water conditioning
Products which are intended to be in intermittent contact with hot water shall undergo the cold application conditioning according to 4.5.5.1. At the conclusion of the cold application conditioning, the products shall be further conditioned in the exposure water(s) selected in 4.5.3 at either 60 ± 2EC (140 ±3EF) or 82 ±2EC (180 ± 3EF) for two consecutive 1 hour ± 5 minute periods. The exposure water shall be decanted and discarded after each 1-hour period. Exposure of the sample according to 4.5.6 shall immediately follow completion of the further conditioning.
NOTE – The stated duration of the conditioning period at the hot temperature does not include any time needed to elevate the product sample or exposure vessel to the required exposure temperature.
4.5.5.2.2 Continuous hot water conditioning
Products which are intended to be in continuous contact with hot water shall be conditioned in the exposure water(s) selected in 4.5.3 at either 60 ± 2EC (140 ± 3EF) for 14 days. During the 14-day period, the exposure water shall be changed at least 10 times with a minimum period of 24 ± 1 hour between water changes. The free available chlorine concentration during the conditioning period shall be 2 mg/L. After the 14-day conditioning period, the exposure water in the product or in the vessel shall be decanted and discarded. Shortened conditioning periods shall be permitted at the request of the manufacturer. Exposure of the sample according to 4.5.6 shall immediately follow conditioning.
NOTE – Table 4.1 provides an example single time point conditioning protocol. Alternate protocols shall be permitted as long as the requirements of 4.5.5.2 are met.
4.5.6 Single time point exposure protocols
Products to be evaluated at a single time point shall be exposed according to the schedule in table 4.2. The first two 24-hour exposure periods shall be optional at the discretion of the manufacturer. A separate sample shall be exposed for each type of exposure water selected in 4.5.3. For each sample, the exposure water shall be of the same pH as the water used for conditioning of the sample.
4.5.6.1 Single time point exposure–cold application
Immediately following conditioning, the product shall be exposed at 23 ± 1°C (73 ± 2°F) according to the schedule in table 4.2.
4.5.6.2 Single time point exposure–hot applications
4.5.6.2.1 Intermittent hot water exposure
Immediately following conditioning, the product shall undergo the cold application exposure according to 4.5.6.1. Prior to the final 16-hour exposure, the product shall be exposed at the selected elevated temperature, either 60±1EC (140 ± 2EF) or 82 ± 1EC (180 ± 2EF), for 0.5 hour ± 5 minutes. The product shall then be exposed at 23 ± 1°C (73 ± 2°F) for the duration of the exposure period. The exposure water shall not be decanted prior to initiation of the final 16-hour exposure.
154.5.6.2.2 Continuous hot water exposure
Immediately following conditioning, the product (in-product exposures) or the exposure vessel (in-vessel exposures) shall be filled with fresh exposure water of the applicable pH (see 4.5.3). The product shall then be exposed at the selected elevated temperature, either 60 ± 1EC (140 ± 2EF) or 82 ± 1EC (180 ± 2EF), according to the schedule in table 4.2.
4.5.7 Multiple time point conditioning/exposure protocols
For the purpose of determining a contaminant leaching rate as a function of time, extractrant water samples shall be collected during the conditioning period of products for which multiple time point exposure has been elected, according to the protocols in 4.5.7.1 and 4.5.7.2. A separate sample shall be conditioned and exposed for each type of exposure water selected in 4.5.3.
4.5.7.1 Cold application
Products which are intended to be in contact with only cold water shall be maintained at 23 ± 1°C (73 ± 2°F) for 19 days. During the 19-day period, the exposure water shall be changed at least 12 times, with a minimum period of 24 ± 1 hour between water changes. At seven of these water changes, extraction water shall be collected for analysis after a 24-hour exposure. For extrapolation and normalization purposes, the number of hours elapsed since the most recent water change (or sample collection) and the number of days elapsed since the initiation of the exposure shall be recorded at the time of each extraction water collection.
NOTE – Table 4.3 provides an example multiple time point conditioning/exposure protocol. Alternate protocols shall be permitted as long as the requirements of 4.5.7.1 are met.
At the discretion of the manufacturer, direct measurement of a Day 90 extraction shall be permitted. The products shall be maintained at 23 ± 1°C (73 ± 2°F). Extraction water shall be collected for analysis at a minimum of two time points: after Day 1 (representing 14 days of conditioning and 1 day of acute exposure), and after the final exposure terminating on Day 90 (representing 14 days of conditioning, 1 day of acute exposure, and 90 days of chronic exposure). The exposure water shall be changed at least 4 days per week during the interval between the initial and final exposures. Exposures which are used for the collection of extractant water for analysis shall not exceed 24 ± 1 hour in duration.
4.5.7.2 Hot applications
4.5.7.2.1 Intermittent hot water exposure
Products which are intended to be in intermittent contact with hot water shall undergo the cold application exposure according to 4.5.7.1. At the initiation of each exposure which will be collected for analysis, the product shall be exposed at the selected elevated temperature, either 60 ± 1EC (140 ± 2EF) or 82 ± 1EC (180 ± 2EF), for 0.5 hour ± 5 minutes. The product shall then be exposed at 23 ± 1°C (73 ± 2°F) for the duration of the exposure period. The exposure water shall not be decanted prior to the completion of the exposure period.
NOTE 1 – Table 4.3 provides an example multiple time point conditioning/exposure protocol. Alternate protocols shall be permitted as long as the requirements of 4.5.7.2.1 are met.
NOTE 2 – The stated duration of the conditioning period at the hot temperature does not include any time needed to elevate the product sample or exposure vessel to the required exposure temperature.
At the discretion of the manufacturer, direct measurement of a Day 90 extraction shall be permitted. At the initiation of each exposure which will be collected for analysis, the products shall be exposed at the selected elevated temperature, either 60 ± 1EC (140 ± 2EF) or 82 ± 1EC (180 ± 2EF), for 0.5 hour ± 5 minutes. The product shall then be exposed at 23 ± 1°&C (73 ± 2°F) for the duration of the exposure period. The exposure
16water shall not be decanted prior to the completion of the exposure period. Extraction water shall be collected for analysis at a minimum of two time points: after Day 1 (representing 14 days of conditioning and 1 day of acute exposure), and after the final exposure terminating on Day 90 (representing 14 days of conditioning, 1 day of acute exposure, and 90 days of chronic exposure). The exposure water shall be changed at least four days per week during the interval between the initial and final exposures. Exposures which are used for the collection of extractant water for analysis shall not exceed 24 ± 1 hour in duration.
4.5.7.2.2 Continuous hot water exposure
Products which are intended to be in continuous contact with hot water shall be maintained at the selected elevated temperature, either 60 ± 1°C (140 ± 2°F) or 82 ± 1°C (180 ± 2°F) for 19 days. During the 19-day period, the exposure water shall be changed at least 12 times with a minimum period, of 24 ± 1 hour between water changes. At seven of these water changes, extraction water shall be collected for analysis after a 24-hour exposure. For extrapolation and normalization purposes, the number of hours elapsed since the most recent water change (or sample collection) and the number of days elapsed since the initiation of the exposure shall be recorded at the time of each extraction water collection.
NOTE – Table 4.3 provides an example multiple time point conditioning/exposure protocol. Alternate protocols shall be permitted as long as the requirements of 4.5.7.2.2 are met.
At the discretion of the manufacturer, direct measurement of a Day 90 extraction shall be permitted. The products shall be maintained at the selected elevated temperature, either 60 ± 1°C (140 ± 2°F) or 82 ± 1°C (180 ± 2°F). Extraction water shall be collected for analysis at a minimum of two time points: after Day 1 (representing 14 days of conditioning and 1 day of acute exposure), and after the final exposure terminating on Day 90 (representing 14 days of conditioning, 1 day of acute exposure, and 90 days of chronic exposure). The exposure water shall be changed at least 4 days per week during the interval between the initial and final exposures. Exposures which are used for the collection of extractant water for analysis shall not exceed 24 ± 1 hour in duration.
4.5.8 Collection and preservation of extraction water
Immediately following exposure, extraction waters collected for analysis shall be poured into previously prepared sample containers for storage until analysis, as specified in annex B, section B.6.
4.6 Analysis
4.6.1 Extraction waters shall be analyzed with the methods listed in annex B, section B.7.
4.6.2 Samples requiring analysis for residual vinyl chloride monomer shall be evaluated according to the method in annex B, section B.7.
4.7 Normalization of contaminant concentrations
4.7.1 General
The concentration of analytes detected in the extraction water shall be multiplied by a calculated normalization factor (NF) to account for differences between laboratory and field surface area-to-volume ratios. The normalization factor shall be based on calculations and assumptions relevant to the end-use of the product.
17The general formula for the derivation of the normalization factor is described in the following equations:
NF = N1 × N2
Where:
SAF = Surface area exposed in the field
SAL = Surface area exposed in the laboratory
VL = Volume of extraction water used in the laboratory
VF(static) = Volume of water to which the product is exposed under static conditions
VF(flowing) = Volume of water to which the product is exposed under flowing conditions during a period of time equivalent to the laboratory test
When the length of the exposure being normalized is other than 16 hours in length, the normalized value shall be adjusted to reflect a 16-hour exposure (e.g. multiply the normalized value by 16/24 when a 24-hour exposure was used.) The nominal diameter of the product shall determine which assumptions are used for normalization (see tables 4.4 and 4.5.) The actual inner diameter of the product shall be used for the normalization calculations of surface area and volume.
NOTE – Adjustment of the normalized contaminant concentration for the duration of the exposure period shall consider the extraction kinetics of the contaminant under evaluation. For contaminants which do not exhibit linear extraction kinetics, adjustment for the duration of exposure shall be done in accordance with the demonstrated kinetics of the contaminant or shall not be applied if this information is not available.
4.7.2 Products installed at regularly repeating intervals
For products installed at regularly repeating intervals (e.g., pipes, fittings), the SAF shall be calculated from the assumed length of pipe corresponding to the segment of the system in which the product is used (e.g., 100 feet of pipe in the service line or 280 feet of pipe in the residence). The VF(static) component of the N1 term shall be the volume of water contained within the assumed length of pipe. For fittings, the actual inner diameter of the pipe used with the fittings shall be used to calculate both SAF and VF(static).
4.7.3 Products not installed at regularly repeating intervals
Products not installed at regularly repeating intervals shall be identified through review of the manufacturer's recommended product end use. For products not installed at regularly repeating intervals (e.g., transition fittings, repair couplings, drop ear elbow fittings, and copper stub outs), the SAF shall be the wetted surface area of a single product. The VF(static) component of the N1 term shall be the volume of water a single product contains when filled to capacity, except that VF(static)shall equal 1 L(0.264 gal) for all products that contain less than 1 L of water when filled to capacity.
4.7.4 Sample calculations for normalization of section 4 products are provided in table 4.6.
4.7.5 Selection of normalization conditions
Pipe and fitting products of nominal diameter greater than or equal to 4 in (10 cm) shall be normalized to the flowing condition. Pipe and fitting products of nominal diameter of less than 4 in (10 cm) shall be normalized to the static condition when the value of N2 is less than or equal to 0.1. Pipe and fitting products of nominal
18diameter of less than 4 in (10 cm) shall be normalized to the flowing condition when the value of N2 is greater than 0.1.
4.7.6 Multiple time point exposure calculations
Laboratory values from each time point at which extractant water was collected (a minimum of five data points shall be required for extrapolation) shall be normalized as indicated in 4.7.1, depending on product end use. A decay curve of these normalized contaminant concentrations in relation to elapsed exposure time shall be plotted. Contaminant concentrations shall be determined for two time point as follows: at Day 1 (representing 14 days of conditioning and 1 day of acute exposure) and at Day 90 (representing 14 days of conditioning, 1 day of acute exposure, and 90 days of chronic exposure) shall be extrapolated from this curve (see 4.5.7).
If direct measurement of a Day 90 exposure has been performed, laboratory values from each time point at which extractant water was collected (a minimum of two time points as defined in 4.5.7.1 and 4.5.7.2) shall be normalized as indicated in 4.7.1, depending on product end use.
4.8 Evaluation of contaminant concentrations.
4.8.1 Contaminants measured in a single time point extraction
For pipe and fitting products, normalized static contaminant concentrations shall be no greater than their respective MCLs or TACs, and normalized flowing contaminant concentrations shall be no greater than their respective SPACs calculated in accordance with annex A.
4.8.2 Contaminants measured in a multiple time point extraction
Normalized Day 1 contaminant concentrations shall not exceed the short-term exposure level (STEL) as defined in annex A, section A.5.
Normalized extrapolated or directly measured Day 90 contaminant concentrations shall not exceed the limits defined in 4.8.1.
4.8.3 Residual vinyl chloride monomer (RVCM)
The average RVCM concentration shall be less than or equal to 3.2 mg/kg as evaluated in the product wall.
19| Conditioning time | Elapsed time | Comment |
|---|---|---|
| 24 ± 1 hour | 1 day | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 2 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 3 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 4 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 72 ± 1 hour | 7 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 8 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 9 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 10 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 24 ± 1 hour | 11 days | Exposure water is decanted and discarded; the exposure vessel or product is refilled with exposure water and conditioning is continued. |
| 72 ± 1 hour | 14 days | Exposure water is decanted and discarded; conditioning is terminated. |
| Exposure time | Elapsed time1) | Comment |
|---|---|---|
| 24 ± 1 hour (optional) | 15 days (optional) | Extraction water is decanted and discarded; the exposure vessel or product is refilled with exposure water and exposure is continued. |
| 24 ± 1 hour (optional) | 16 days (optional) | Extraction water is decanted and discarded; the exposure vessel or product is refilled with exposure water and exposure is continued. |
| 16 hours | 17 days (15 days if the two optional exposure periods are not elected) | Extraction water is collected for analysis. |
| 1) Elapsed time indicated includes the 14 days of conditioning preceding the exposure. | ||
| Exposure time | Elapsed time | Sample collection |
|---|---|---|
| 24 ± 1 hour | 1 day | Extraction water is collected for analysis at completion of the exposure period; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 2 days | Extraction water is collected for analysis at completion of the exposure period; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 3 days | Extraction water is decanted and discarded; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 4 days | Extraction water is collected for analysis at completion of the exposure period; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 72 ± 1 hour | 7 days | Extraction water is decanted and discarded; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 8 days | Extraction water is collected for analysis at completion of the exposure period; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 9 days | Extraction water is decanted and discarded; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 10 days | Extraction water is collected for analysis at completion of the exposure period; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 96 ± 1 hour | 14 days | Extraction water is decanted and discarded; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 15 days | Extraction water is collected for analysis at completion of the exposure period; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 72 ± 1 hour | 18 days | Extraction water is decanted and discarded; the product or exposure vessel is refilled with exposure water and the exposure is continued. |
| 24 ± 1 hour | 19 days | Extraction water is collected for analysis at completion of the exposure period; the exposure is terminated. |
| Product nominal diameter | Assumptions | Exposure type | N1 | N2 (flowing condition) |
|---|---|---|---|---|
| nominal $ 10 cm (4 in) | – water is exposed to the same material from the treatment plant to the service line – a 16-hour exposure period is evaluated |
in-product | 1 | 1 |
| in-vessel | calculated according to 4.7.1 | 1 | ||
| 10 cm (4 in) > nominal ≥ 1.3 cm (½ in) | – a 16-hour exposure period is evaluated – residential water usage is 681 L (180 gal) per 24 hours –100 feet of service line from water main to residence |
in-product | 1 | calculated according to 4.7.1 |
| in-vessel | calculated according to 4.7.1 | calculated according to 4.7.1 | ||
| nominal < 1.3 cm (½ in) | – a maximum run of 25 ft (7.6 m) of small diameter product is installed – for products with an internal volume less than 1L, VF(static) is set equal to 1 L – a 16-hour exposure period is evaluated – residential water usage is 681 L (180 gal) per 24 hours – 280 feet per residence (140 feet each for hot and cold sides) |
in-product | 1 | calculated according to 4.7.1 |
| in-vessel | calculated according to 4.7.1 | calculated according to 4.7.1 |
| Product nominal diameter | Assumptions | Exposure type | N1 | N2(flowing condition) |
|---|---|---|---|---|
| nominal $ 10 cm (4 in) | – water is exposed to the same material from the treatment plant to the service line – fittings represent 2% of the distribution system surface area – a 16-hour exposure period is evaluated |
in-product | 0.02 | 1 |
| in-vessel | calculated according to 4.7.1 and multiplied by 0.02 | 1 | ||
| 10 cm (4 in) > nominal ≥ 1.3 cm (½ in) | – fittings represent 2% of the piping system for products 4 in > nominal $ 1 in (rigid and flexible systems) – fittings represent 6% of the piping system surface area for products 1 in> nominal ≥ ½ in (rigid systems)1) – fittings represent 3% of the piping system surface area for products 1 in > nominal ≥ ½ in (flexible systems)1) – a 16-hour exposure period is evaluated – residential water usage is 681 L (180 gal) per 24 hours – 100 ft of service line from water main to residence |
in-product | 0.02, 0.06, or 0.03 depending on product diameter and end use (flexible or rigid system) | calculated according to 4.7.1 |
| in-vessel | calculated according to 4.7.1 and multiplied by 0.02, 0.06, or 0.03 depending on product diameter and end use (flexible or rigid system) | calculated according to 4.7.1 | ||
| nominal < 1.3 cm (½ in) | – a maximum run of 7.6 m (25 ft) of small diameter product is installed – fittings represent 6% of the residential system surface area for rigid piping systems1) – fittings represent 3% of the residential system surface area for flexible piping systems1) – a 16-hour exposure period is evaluated – residential water usage is 681 L (180 gal) per 24 hours – 280 ft of pipe per residence (140 ft each for hot and cold sides) |
in-product | 0.06 or 0.03 depending on product end use (flexible or rigid system) | calculated according to 4.7.1 |
| in-vessel | calculated according to 4.7.1 and multiplied by 0.06 or 0.03 depending on product end use (flexible or rigid system) | calculated according to 4.7.1 | ||
| 1) For products which may be used with either rigid or flexible systems, fittings shall be assumed to represent 6% of the piping system surface area. | ||||
| In-product exposure of a 1 ft length of 6 in i.d. pipe | |
| Parameters: SAF = 226 in2 (1 459 cm2) SAL = 226 in2 (1 459 cm2) VF(static) = 1.5 gal (5.6 L) VL = 1.5 gal (5.6 L) |
 |
| In-vessel exposure of a 1 in i.d. pipe | |
| Parameters: SAF/ VF(static) = 924 in2/1 gal (1 575 cm2/1 L) SAL = 247 in2 (1 594 cm2) VL = 0.2 gal (0.8 L) |
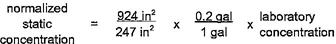 |
| In-product exposure of a 25 ft length of ¼ in i.d. pipe | |
| Parameters: SAF = 235.6 in2 (1 520 cm2) SAL = 235.6 in2 (1 520 cm2) VF(static) = 0.064 gal (0.24 L) – default to 0.26 gal (1 L) VL = 0.064 gal (0.24 L) |
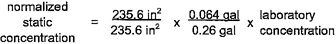 |
| In-product exposure of a 10 in long 6 in i.d. fitting | |
| Parameters: SAF = 188.5 in2 (1 216.1 cm2) SAL = 188.5 in2 (1 216.1 cm2) VF(static) = 1.2 gal (4.6 L) VL = 1.2 gal (4.6 L) |
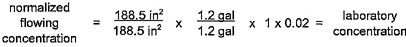 |
| In-vessel exposure of a ½ in i.d. fitting used with flexible piping systems | |
| Parameters: SAF/VF(static) = 1 885 in2/1 gal (3 040 cm2/1 L) SAL = 247 in2 (1 594 cm2 VL = 0.2 gal (0.8 L) |
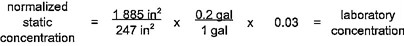 |
| In-vessel exposure of a ¼ in i.d. fitting used with rigid piping systems | |
| Parameters: SAF/VF(static) = 908 in2/1 gal (1 523 cm2/1 L) SAL = 865 in2 (5 581 cm2) VF(static) = 0.064 gal (0.24 L) – default to 0.26 gal (1 L) VL = 0.4 gal (1.3 L) |
 |
| In-vessel exposure of a ½ in i.d. fitting used as a repair coupling | |
| Parameters: SAF/VF(static) = 1885 in2/1 gal (3040 cm2/1 L) VF(static) = 0.0009 gal (0.003 L) – default to 0.26 gal (1 L) SAL = 865 in2 (5 581 cm2) VL = 0.4 gal (1.3 L) |
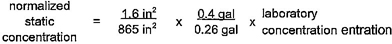 |
|
|
5.1 Scope
The requirements of this section apply to products and materials intended to form a barrier providing containment of drinking water or to prevent drinking water contact with another surface. The products and materials which are covered include, but are not limited to: non-residential storage tanks, coatings, paints, linings and liners, bladders, diaphragms, and constituents of concrete and cement-mortar (e.g., portland and blended hydraulic cements, admixtures, sealers, and mold release agents). These products and materials can be field-applied, factory-applied, precast, or cast in place.
5.2 Definitions
5.2.1 admixture: A material other than water, aggregates, hydraulic cement, and fiber reinforcement used as an ingredient of concrete or mortar and added to the batch immediately before or during its mixing.
5.2.2 aggregate: Granular material, such as sand, gravel, or crushed stone used with a cementing medium to form hydraulic-cement concrete or mortar.
5.2.3 barrier material: A material in contact with drinking water that serves a containment or separation purpose.
5.2.4 blended hydraulic cement: A hydraulic cement consisting of two or more inorganic constituents (at least one of which is not portland cement or portland cement clinker) which separately or in combination contribute to the strength-gaining properties of the cement.
5.2.5 coating/paint: A material applied to a surface where a direct bond to the substrate is formed.
5.2.6 concrete: A composite material that consists essentially of a binding medium within which are embedded particles or fragments of aggregate; in hydraulic-cement concrete, the binder is formed from a mixture of hydraulic cement and water.
5.2.7 diaphragm/bladder: A flexible membrane that separates the surrounding media from the drinking water.
5.2.8 form/mold release agent: A material applied to the inside of a form or mold used to cast concrete or cement-mortar which prevents adhesion of the concrete or cement-mortar to its surface.
5.2.9 hydraulic cement: A cement that sets and hardens by chemical interaction with water and that is capable of doing so under water.
5.2.10 liners/linings: Prefabricated materials applied, bonded, or attached to a surface that is subject to direct/indirect contact with drinking water.
5.2.11 mortar: A mixture of water, cement, and sand.
5.2.12 portland cement: A hydraulic cement (usually containing calcium sulfate) produced by pulverizing portland cement clinker (a partially fused substance consisting primarily of hydraulic calcium silicates).
5.2.13 sealer: A liquid that is applied as a coating to the surface of hardened concrete or cement-mortar, either to prevent or decrease the penetration of liquid or gaseous media during service exposure.
255.3 General requirements
5.3.1 Product labeling
Products or product containers shall be marked and include, at a minimum, product identification, batch number or date of manufacture. When it is not feasible to mark the product or material, the manufacturer shall maintain identification records.
5.3.2 Paints and coatings
For all paints and coatings, the manufacturer shall submit detailed use instructions. Use instructions shall specify the appropriate preparation and application procedures, including order of application for multiple layer systems, substrate preparation (including use of specific primer), subcomponent mixing, induction time, thinning, application method, application thickness(es), curing schedule, and final cure time prior to water immersion.
Coating systems that are composed of multiple products (e.g., primer, intermediate coat(s), and top coat, including any thinners) shall be evaluated as an applied system.
5.4 Sample requirements
When required for evaluation, a sample of the product or material equivalent to that used in field applications shall be obtained.
A single sample can represent a product line of similar formulations (e.g., different colors of the same coating product line) when:
– the sample selected for testing contains all of the formulation ingredients of toxicological concern (see section 3.2) at concentrations equal to or greater than the products it is selected to represent; and
– product application conditions for the sample selected for testing (e.g., application thickness(es), cure times, solvent concentrations) are equal to or more severe than the products it is selected to represent; and
– for multiple component formulations, the mixing ratio(s) of the selected sample is(are) identical to that of the products it represents.
5.4.1 Cement samples
Cement samples, weighing a minimum of 9 kg (20 lbs), shall be collected in accordance with the applicable sections of ASTM C 183. To minimize contamination, all sample collection tools shall be cleaned and wiped with isopropyl alcohol before use. Collected samples shall be placed in moisture-proof containers. To minimize organic contamination, sample containers shall not be filled near a running motor or any type of exhaust system.
5.4.2 Concrete cylinder samples
Concrete test cylinders for the evaluation of cast-in-place or precast concrete structures shall be submitted with specific information on the composition of the concrete mix design for the specific installation, including the specific sources of cement, aggregate, admixtures, and any other additives. Specific information on the tank dimensions and water storage capacity shall also be provided. Concrete batch tickets, collected at the site of production, shall serve as evidence of the concrete mix actually used in the structure being evaluated.
265.4.3 Other barrier materials
Samples of barrier materials shall be collected at the point of manufacture.
5.5 Extraction procedures
5.5.1 Analytical summary
An analytical summary shall be prepared for each product. The analytical summary shall consist of the formulation-dependent analytes identified through the formulation review (see 3.2) and the applicable product-specific analytes listed in table 3.1.
5.5.2 Preparation of test samples
5.5.2.1 In all cases, test samples shall be prepared such that a minimum surface area to volume ratio of 50 cm2/L (29 in2/gal) is achieved during the exposure, and so that the entire surface to be exposed is covered by exposure water. Samples shall be rinsed with cold tap water and then in deionized water (ASTM D 1193 Type II), unless manufacturer’s instructions direct otherwise.
5.5.2.2 Field-applied paint and coating systems
These products shall be applied in accordance with the manufacturer’s published use instructions (see 5.3.2) under the supervision of the certifying agency. Products shall be applied to a glass slide when appropriate. Products requiring a reactive substrate shall be applied to the appropriate alternate substrate. Coating products shall be applied using application conditions as specified by the manufacturer in the product use instructions, e.g., the highest recommended percentage of thinner, the shortest curing period between coats or layers, the recommended film thickness per coat, and the shortest final curing period prior to immersion.
Multiple layer paint and coating systems which require the application of distinct coating product formulations in sequence shall be applied in a stepped manner so as to expose all layers. Multiple coats of the same product (of the same color) applied in sequence shall not constitute multiple layers and shall not be applied in a stepped manner. Multiple coats of the same product (of different colors) applied in sequence shall not constitute multiple layers and shall not be applied in a stepped manner, unless deemed necessary by the testing laboratory to address potential health effects concerns from the differences in color formulations. Stepped coating systems shall be applied per the dimensions in table 5.1.
5.5.2.3 Factory-applied or cured systems
Paint and coating systems requiring factory application, factory curing, or both, shall be prepared in accordance with the manufacturer’s published use instructions under the supervision of the certifying agency. These products shall be applied in accordance with the manufacturer’s published use instructions (see 5.3.2). Products shall be applied to a glass slide when appropriate. Products requiring a reactive substrate shall be applied to the appropriate alternate substrate. Coating products shall be applied using application conditions as specified by the manufacturer in the product use instructions, e.g., the highest recommended percentage of thinner, the shortest curing period between coats or layers, the recommended film thickness per coat, and the shortest final curing period prior to immersion.
Multiple layer paint and coating systems which require the application of distinct coating product formulations in sequence shall be applied in a stepped manner so as to expose all layers. Multiple coats of the same product (of the same color) applied in sequence shall not constitute multiple layers and shall not be applied in a stepped manner. Multiple coats of the same product (of different colors) applied in sequence shall not constitute multiple layers and shall not be applied in a stepped manner, unless deemed necessary by the
27testing laboratory to address potential health effects concerns from the differences in color formulations. Stepped coating systems shall be applied per the dimensions in table 5.1.
NOTE – It is recognized that a coating system may be applied using a combination of factory and field application techniques. This is considered acceptable as long as the coating system is tested to the manufacturer's recommended application conditions, as specified in 5.5.2.2 and 5.5.2.3.
5.5.2.4 Products requiring cement mortar cubes
Test sample mortar cubes shall be prepared in accordance to the applicable sections of ASTM C 109. Mix water shall meet ASTM D 1193 Type II requirements. Sand shall be washed in accordance with the procedures in ASTM C 778. Mixing tools and other items coming into contact with the mortar shall be washed with soap and water, rinsed with tap water, rinsed with ASTM D 1193 Type II water, and rinsed with isopropyl alcohol. The mortar shall be placed in polyethylene or polypropylene lined molds; no form release agents shall be used. Specimens shall be removed from the molds after 24 hours and placed in glass or polyethylene beakers and covered with in inverted watch glass supported on glass Rebel hooks (or other devices to prevent air seal of the vessel) and placed for 28 days ± 12 hours, or fewer as specified by the manufacturer, in a moist cabinet meeting the requirements of ASTM C 511. The specimens shall be removed from the moist cabinet and air dried at 23 ± 2°C (73 ± 4EF) and 50 ± 2% relative humidity for 7 days.
5.5.2.4.1 Portland and hydraulic cements
Test cubes for portland and blended hydraulic cements shall be prepared in accordance with 5.5.2.4.
5.5.2.4.2 Admixtures
These products shall be added to the cement – mortar or concrete mixture using the manufacturer's highest recommended admixture dosage. The test samples shall be prepared as described in 5.5.2.4.
5.5.2.4.3 Sealers
These products shall be applied per manufacturer's recommendations to the test cubes prepared in accordance with 5.5.2.4. The coated cubes shall be allowed to cure for the manufacturer’s recommended time period.
5.5.2.4.4 Form and mold release agents
These products shall be applied per manufacturer specifications to the mold used during the preparation of the test cubes (see 5.5.2.4).
5.5.2.5 Concrete water storage tanks
Concrete test cylinders (4" × 8") shall be prepared according to ASTM C 31 or ASTM C 192, and moist cured in an ASTM C 511 cabinet for a minimum of three days. Cylinder molds shall be manufactured of virgin materials free of detectable concentrations of any interfering contaminants.
5.5.3 Exposure water
Exposure water selection shall be determined by the analytes of interest identified on the analytical summary (see 5.5.1). Exposure water(s) shall be selected in accordance with annex B, section B.2.5.
285.5.4 Conditioning
Test samples shall be conditioned immediately after curing. This conditioning procedure simulates the disinfection of water storage tanks prior to placing into service. The method described is based on Method 2 of AWWA Standard C652-92.
1) prepare 200 mg/L available chlorine solution using sodium hypochlorite (NaOCl – reagent grade or equivalent);
2) using a spray bottle, spray the previously rinsed test samples, wetting all surfaces to be exposed;
3) let the test samples stand for at least 30 minutes; and
4) place the test samples in racks, rinse with cold tap water, and rinse with ASTM D 1193 Type II deionized water.
5.5.5 Exposure protocols
For all test samples, exposure shall commence immediately following the conditioning step. If immediate exposure is not possible, the test samples shall be dried in a laminar flow hood and exposed within four hours. Successful evaluation at an elevated exposure temperature shall preclude testing at a lower exposure temperature. A separate sample shall be exposed for each type of exposure water selected in 5.5.3.
The exact surface area-to-volume ratio achieved during the exposure shall be recorded.
5.5.5.1 Cold application
Cold application product samples, as designated by the manufacturer, shall be placed in an exposure vessel and completely covered with exposure water of the applicable pH (see 5.5.3). The exposure vessel shall be placed in a 23 ± 2°C (73 ± 4°F) environment for the duration of the exposure period.
5.5.5.2 Domestic hot application
Products which are intended for domestic hot applications, as designated by the manufacturer, (e.g., for use in single family dwellings) shall be placed in an exposure vessel and completely covered with exposure water of the applicable pH (see 5.5.3). The exposure vessel shall be placed in a 60 ± 2°C (140 ± 4°F) environment for the duration of the exposure period.
5.5.5.3 Commercial hot application
Products which are intended for commercial hot applications, as designated by the manufacturer, (e.g., for use in multiple family dwellings, restaurants, hospitals) shall be placed in an exposure vessel and completely covered with exposure water of the applicable pH (see 5.5.3). The exposure vessel shall be placed in a 82 ± 2°C (180 ± 4°F) environment for the duration of the exposure period.
5.5.5.4 Single time point exposure protocol
When normalized contaminant concentrations from the product are expected to be less than their acceptable concentrations (see annex A) when tested at a single time point (e.g., flexible membrane liners), the product shall be exposed according to the protocol in table 5.2. Extraction water samples shall be collected at the conclusion of the final exposure period.
295.5.5.5 Multiple time point exposure protocol
When the normalized concentration of a contaminant exceeds, or is expected to exceed, its acceptable concentration (see annex A) when evaluated as a single time point (see 5.5.5.4), determination of the contaminant leaching rate as a function of time shall be considered. The relationship between contaminant concentration(s) and time shall be determined and plotted using a minimum of five data points. Table 5.3 summarizes the multiple time point exposure sequence. For contaminants of interest which do not require over time testing, extraction water shall be collected following the third exposure period (elapsed time five days).
5.5.6 Collection and preservation of extraction water
Immediately following the exposure period, the extraction water shall be poured into previously prepared sample containers for storage as detailed in annex B, section B.6 until analysis. Extraction water for solvent analysis shall be collected in a sample bottle containing sodium thiosulfate in a quantity sufficient to neutralize any residual chlorine, if applicable.
5.6 Analysis of extraction water
Extraction waters shall be analyzed with the methods listed in annex B, section B.8.
5.7 Normalization
5.7.1 Normalization for tanks/storage vessels
5.7.1.1 The following equation shall be used to calculate the normalized concentration of each contaminant for tanks or other storage vessels:
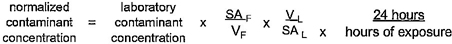
Where:
SAF/VF = Surface area to volume ratio for the specified tank capacity, as defined in table 5.4
SAL = Surface area exposed in the laboratory
VL = Volume of extraction water used in the laboratory
When the length of the exposure being normalized is other than 24 hours in length, the normalized value shall be adjusted to reflect a 24 hour exposure.
Products used as barriers for tanks or storage vessels shall use the surface area to volume ratios shown in table 5.4. Surface area to volume ratios for products used as barriers in tanks or storage vessels with a capacity other than those shown in table 5.4 shall be determined on a case by case basis, as described in 5.7.1.2.
5.7.1.2 Calculation of the surface area to volume ratio for tanks or storage vessels
The following assumptions shall be used in determining the surface area to volume ratio for each nominal tank capacity:
– the tank has a smooth interior surface;
– the tank is cylindrical in shape;
30– the tank is installed in a vertical position; and
– the roof or top of the tank is not in contact with drinking water.
The following equation shall be used to calculate the surface area to volume ratio for tanks or storage vessels of capacities which do not appear in table 5.4:
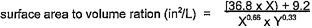
Where:
X = the length/diameter ratio of the tank or storage vessel
Y = the volume (in gallons) of the tank or storage vessel
5.7.2 Normalization for all other end uses
For barrier materials which have end uses other than tanks or storage vessels, normalization shall be performed using the following equation, or to the normalization requirements of the section of this standard which addresses the specific end use of the barrier material.
NF = N1 × N2
Where:
SAF = Surface area exposed in the field
SAL = Surface area exposed in the laboratory
VL = Volume of extraction water used in the laboratory
VF(static) = Volume of water to which the product is exposed under static conditions
VF(flowing) = Volume of water to which the product is exposed under flowing conditions during a period of time equivalent to the laboratory test.
When the length of the exposure being normalized is other than 24 hours in length, the normalized value shall be adjusted to reflect a 24 hour exposure (e.g., multiply the normalized value by 24/72 when a three day exposure was used).
5.7.3 Over time exposure calculations
Laboratory values from each time point for which extractant water was collected (minimum of five data points required) shall be normalized as indicated in 5.7.1 or 5.7.2, depending on product end use. A decay curve of these normalized contaminant concentrations in relation to elapsed exposure time shall be plotted. A contaminant concentration at Day 90 of exposure shall be extrapolated from this data.
31NOTE – Day 1 is defined as the time point at which extractant water for all contaminants is collected for analysis (five days of elapsed time). Day 90 is defined as 90 days following this time point (95 days of elapsed time).
5.8 Evaluation of contaminant concentrations
5.8.1 Contaminants measured at a single time point
Normalized contaminant concentrations shall be no greater than their respective MALs determined in accordance with annex A.
5.8.2 Contaminants measured over time
Normalized Day 1 contaminant concentrations shall not exceed the short-term exposure level (STEL) as defined in annex A, A.5. Extrapolated Day 90 contaminant concentrations shall not exceed their respective MALs determined in accordance with annex A.
32| Number of layers in system | Layer | Panel surface area exposed for each layer |
|---|---|---|
| one layer | — | entire panel |
| two layer | primer layer top layer |
1/3 2/3 |
| three layer | primer layer intermediate layer top layer |
1/6 1/3 1/2 |
| four layer | primer layer first intermediate layer second intermediate layer top layer |
1/12 1/6 1/4 1/2 |
| NOTE –A layer is one or more coats of the same coating material. | ||
| Length of exposure | Elapsed time | Sample collection |
|---|---|---|
| 24 ± 1 hour | 1 day | discard extractant water and refill |
| 24 ± 1 hour | 2 days | discard extractant water and refill |
| 48 ± 4 hours | 4 days | discard extractant water and refill |
| 24 ± 1 hour | 5 days | extractant water collected for analysis at conclusion of exposure period |
| NOTE – Sample exposures are sequential: decant and discard extraction water, refill container, and continue exposure. | ||
| Length of exposure | Elapsed time | Sample collection |
|---|---|---|
| 24 ± 1 hour | 1 day | extractant water collected for analysis |
| 24 ± 1 hour | 2 days | extractant water collected for analysis |
| 48 ± 4 hours | 4 days | discard extractant water and refill |
| 24 ± 1 hour | 5 days | extractant water collected for analysis |
| 6 ± 1 day | 11 days | discard extractant water and refill |
| 24 ± 1 hour | 12 days | extractant water collected for analysis |
| 6 ± 1 day | 18 days | discard extractant water and refill |
| 24 ± 1 hour | 19 days | extractant water collected for analysis |
| 6 ± 1 day | 25 days | discard extractant water and refill |
| 24 ± 1 hour | 26 days | extractant water collected for analysis |
| 6 ± 1 day | 32 days | discard extractant water and refill |
| 24 ± 1 hour | 33 days | extractant water collected for analysis |
| NOTE – Sample exposures are sequential: decant required volume for analysis when indicated, discard any remaining extraction water, refill container, and continue exposure. | ||
| Nominal capacity (gal) | Surface area (ft2)1 | Length/diameter ratio | Surface area to volume ratio (in2/1 L) |
|---|---|---|---|
| 5 | 5 | 5.0 | 38.5 |
| 10 | 8 | 5.0 | 30.6 |
| 25 | 14.8 | 5.0 | 22.5 |
| 50 | 20.5 | 3.0 | 15.6 |
| 75 | 26.8 | 3.0 | 13.6 |
| 100 | 32.5 | 3.0 | 12.4 |
| 200 | 51 | 2.9 | 9.7 |
| 300 | 66 | 2.7 | 8.4 |
| 400 | 78.9 | 2.6 | 7.5 |
| 500 | 90.4 | 2.5 | 6.9 |
| 600 | 101 | 2.3 | 6.4 |
| 700 | 110 | 2.2 | 6.0 |
| 800 | 118.7 | 2.1 | 5.7 |
| 900 | 126.5 | 1.9 | 5.4 |
| 1000 | 133.6 | 1.8 | 5.1 |
| 1500 | 175 | 1.8 | 4.4 |
| 2000 | 212 | 1.8 | 4.0 |
| 3000 | 278 | 1.8 | 3.5 |
| 4000 | 337 | 1.8 | 3.2 |
| 5000 | 391 | 1.8 | 3.0 |
| 6000 | 441 | 1.8 | 2.8 |
| 7000 | 489 | 1.8 | 2.7 |
| 8000 | 535 | 1.8 | 2.5 |
| 9000 | 578 | 1.8 | 2.44 |
| 10,000 | 620 | 1.8 | 2.4 |
| 20,000 | 985 | 1.8 | 1.9 |
| 30,000 | 1290 | 1.8 | 1.6 |
| 40,000 | 1563 | 1.8 | 1.5 |
| 50,000 | 1814 | 1.8 | 1.4 |
| 60,000 | 2048 | 1.8 | 1.3 |
| 70,000 | 2270 | 1.8 | 1.23 |
| 80,000 | 2481 | 1.8 | 1.2 35 |
| 90,000 | 2684 | 1.8 | 1.13 |
| 100,000 | 2879 | 1.8 | 1.1 |
| 200,000 | 4570 | 1.8 | 0.9 |
| 250,000 | 5303 | 1.8 | 0.8 |
| 500,000 | 8418 | 1.8 | 0.6 |
| 750,000 | 11,031 | 1.8 | 0.56 |
| 1,000,000 | 13,363 | 1.8 | 0.5 |
| 1,500,000 | 17,511 | 1.8 | 0.44 |
| 2,000,000 | 21,213 | 1.8 | 0.4 |
| 5,000,000 | 39,075 | 1.8 | 0.3 |
| 7,500,000 | 51,203 | 1.8 | 0.26 |
| 10,000,000 | 62,028 | 1.8 | 0.2 |
| 1) Surface area calculations include the sides and bottom of the tank, but not the area of the roof. | |||
6.1 Coverage
This section covers materials that join or seal pipes and related products (e.g., tanks), protective (barrier) materials, and mechanical devices which contact drinking water.
6.2 Definitions
6.2.1 fluxes: Formulations intended to remove traces of surface oxides, promote wetting, and protect the surfaces to be soldered or brazed from oxidation during heating.
6.2.2 gaskets and sealing materials: Materials used to fill a hole or joint to prevent leakage.
6.2.3 joining materials: Materials that form a bond when used to put parts together.
6.2.4 Iubricant: Any substance interposed between two surfaces for the purpose of reducing the friction or wear between them.
6.3 Material and extraction testing requirements
Samples for testing shall be prepared as specified by the manufacturer's written instructions and exposed as outlined in annex B. Any contaminants extracted shall have normalized concentrations no greater than those limits specified in annex A.
6.4 Items of special significance
The manufacturer shall supply written information relative to the product's intended end uses and applications.
377.1 Scope
The requirements in this section apply to process media products intended for the reduction of dissolved or suspended materials present in drinking water. The products that are covered include, but are not limited to, process media used in the following processes: ion exchange, adsorption, oxidation, aeration, and filtration.
7.2 Definitions
7.2.1 adsorption: The retention of a gas, liquid, solid, or dissolved material into the surface of a solid.
7.2.2 adsorption media: A process media material upon which a gas, liquid, solid, or dissolved material will be retained.
7.2.3 aeration: The process of bringing water into contact with air in order to expedite the transfer of gas between the two phases.
7.2.4 aeration packing media: Media used in aerators to increase the surface area of the liquid being processed resulting in increased liquid to air contact and improved gas transfer.
7.2.5 filtration: The process of passing a dilute liquid suspension through filter media to reduce the concentration of suspended or colloidal matter.
7.2.6 filtration media: Process media through which a liquid is passed for the purpose of filtration.
7.2.7 ion exchange: A chemical process in which ions are reversibly interchanged between a liquid and a solid.
7.2.8 ion exchange resins: Process media consisting of insoluble polymers having functional groups capable of exchanging ions.
7.2.9 low density process media: Process media such as diatomaceous earth, perlite, or other media which have a bulk density of less than 500 g/L and are used for filtration purposes.
7.2.10 oxidative media: Process media which chemically facilitates oxidation on the media surface and thereby enhances removal of ions from water.
7.2.11 process media: Water insoluble material used to reduce the concentration of dissolved or suspended substances in water through such operations as ion exchange, aeration, adsorption, oxidation, and filtration.
7.2.12 reductive media: Process media which chemically facilitates reduction on the media surface and thereby enhances removal of ions from water.
7.3 General requirements
7.3.1 Manufacturer use instructions
All process media products shall be accompanied by detailed manufacturer use instructions that shall also appear on the product packaging or other technical literature. For process media products which are dosed (e.g. powdered activated carbon), use instructions shall include the maximum dose at which the product can be acceptably used (as determined by evaluation to the requirements of this section).
387.3.2 Product labeling
Process media product containers shall facilitate traceability to the production location and shall, at a minimum, contain the following information:
– manufacturer's name and address;
– production location identifier;
– product identification (product type and, when applicable, trade name);
– net weight;
– when applicable, mesh or sieve size;
– lot number, and
– when appropriate, special handling, storage, and use instructions.
7.3.3 Product line evaluation
When a line of products is manufactured to the same material formulation and contains identical ingredients, product evaluation shall be preferentially conducted on the product form that has the hightest surface area to be considered to have met the requirements of this section when a higher surface area to volume ratio product, volume ratio (smallest particle size). Products of a lower surface area to volume ratio (larger particle size) shall be considered to have met the requirements of this section when a higher surface area to volume ratio product, belonging to the same line of products and having an identical use, has been demonstrated to meet the requirements of this section.
7.4 Sample requirements
A representative sample of the media shall be reduced to three test samples, each of a sufficient quantity for the extraction procedures described in section 7.5. The three test samples shall be placed and stored in airtight, mositure proof, sealed glass containers. If a glass container is inappropriate, containers made from some other inert material recommended by the manufacturer shall be used. Each container shall be clearly labeled with product name, type of sample, manufacturer name, sampling data, production location, lot number, and the name of the individual who collected the sample. One sample shall be used for exposure and analysis; the remaining two samples shall be retained for re-evaluation purposes.
7.5 Extraction procedures
7.5.1 Analytical summary
An analytical summary shall be prepared for each product. The analytical summary shall consist of the formulation-dependent analytes identified in accordance with section 3.3 and the applicable product-specific minimum test batteries listed in table 7.1.
7.5.2 Wetting
Process media that receive conditioning shall be immersed completely (wetted) in tap water prior to conditioning and exposure. The weight of the sample to be wetted shall be at least equal to the amount of media required to perform the exposure at the specified weight to volume ratio (see table 7.3 and section 7.5.5)
39NOTE – For example, a media for which 2 L of extractant water is required to perform the selected analyses, and the media is exposed at 25 g per L, a minimum of 50 g of media is wetted.
For low density process media, 0.5 L of the process media shall be wetted; the weight of this volume of media shall be measured and recorded prior to wetting.
Following the specified wetting period, the sample shall be completely drained and the water discarded.
7.5.2.1 Granular activated carbon
Granular activated carbon (GAC) test samples shall be wetted out for 16 ± 1 hour.
7.5.2.2 Other process media products
All other process media that receive conditioning shall be wetted out for 60 ± 10 minutes.
7.5.3 Conditioning (backwashing)
7.5.3.1 Filtration and adsorption media
Wetted filtration or absorption media, excluding diatomaceous earth, perlite, and powdered activated carbon (PAC) products, and other media of < 0.25 mm diameter, shall be placed in a conditioning chamber (a glass column having a minimum inner diameter of 2 inches). The amount of media conditioned shall be sufficient to meet or exceed its specific weight per volume ratio (see table 7.2) and to generate sufficient exposure water to complete the selected analyses. Tap water shall be directed slowly upward through the conditioning system until the entire amount of media is flooded. The media shall be backwashed at a flow rate that fluidizes the media or attains sufficient transport velocities to remove extraneous particulate matter; the maximum wetted media expansion rates for various process media products are indicated in table 7.3. Filtration and adsorption media shall be subjected to the prescribed backwash for 30 ± 2 minutes.
7.5.3.2 Diatomaceous earth, perlite, PAC, and other process media
Diatomaceous earth, perlite, PAC, and all other process media having functions other than filtration or adsorption shall not be conditioned unless manufacturer use instructions stiplulate a specific conditioning protocol.
7.5.3.3 Special post-conditioning procedures for sand and anthracite products
Upon completion of the backwash, 1% to 1.5% of the sand or anthracite column (by height) shall be scraped away and discarded.
7.5.4 Exposure water
7.5.4.1 Adsorption media
Adsorption media shall be exposed in a pH 5 sodium dihydrogen phosphate buffer, prepared by mixing 0.1 M NaH2PO4, 0.04 M MgCl2, and ASTM D 1193 Type II deionized water at a ratio of 1:1:18, respectively.
7.5.4.2 All other process media
All other process media shall be exposed in ASTM D 1193 Type II deionized water.
407.5.5 Exposure protocols
Table 7.3 contains the weight per volume ratios for exposure of process media.
7.5.5.1 Adsorption media
7.5.5.1.1 Media of < 0.25 mm in diameter
Immediately after completion of wetting, the media sample shall be exposed in an appropriately sized vessel. The amount of media exposed per volume of exposure water (section 7.5.4.1) shall be sufficient to meet or exceed its specific weight per volume ratio in table 7.2 and to generate sufficient exposure water to complete the selected analysis. The vessel shall be covered and placed on a magnetic stirrer for 1 hour ±5 minutes. Immediately following the exposure period, the liquid portion of the exposure shall be passed through a Whatman # 41 filter and a 0.45 µ filter and the resulting filtrate shall be collected. The solid portion of the exposed sample remaining on the filter shall be dried and weighed and shall be used to calculate the evaluation dose.
7.5.5.1.2 Media of $ 0.25 mm in diameter
Immediately after completion of conditioning, the media sample shall be exposed in an appropriately sized vessel. The amount of media exposed per volume of exposure water (section 7.5.4.1) shall be sufficient to meet or exceed its specific weight per volume ratio in table 7.2 and to generate sufficient exposure water to complete the selected analyses. The contents of the vessel shall be mixed to ensure that the entire sample is in contact with the exposure water. The vessel shall be sealed with PTFE, and the sample shall be exposed according to the schedule outlined in table 7.4. The weight to volume ratio shall be recorded at the time of exposure and shall represent the evaluation dose.
7.5.5.2 Filtration media, ion exchange resins, synthetic media, and all other process media
Immediately after completion of wetting, or conditioning if applicable, the media sample shall be exposed in an appropriately sized vessel. The amount of media exposed per volume of exposure water (section 7.5.4.2) shall be sufficient to meet or exceed its specific weight per volume ratio in table 7.2 and to generate sufficient exposure water to complete the selected analyses. The contents of the vessel shall be mixed to ensure that the entire sample is in contact with the exposure water. The vessel shall be sealed with PTFE, and the sample shall be exposed according to the schedule outlined in table 7.4. The weight to volume ratio shall be recorded at the time of exposure and shall represent the evaluation dose.
7.5.5.3 Aeration packing media
Aeration packing media shall be exposed in appropriately sized vessels at a surface area to volume ratio greater than or equal to its manufacturer's recommended field surface area to volume ratio, and in a volume of exposure water sufficient to complete the selected analyses. The vessel shall be sealed with PTFE, and the sample shall be exposed according to the schedule outlined in table 7.4.
NOTE – The volume of extraction water can be proportionately increased if an additional amount of media was prepared in order to complete the selected analyses.
7.5.6 Collection and preservation of extraction water
Immediately following exposure, extraction waters shall be poured into previously prepared sample containers for storage until analysis, as specified in annex B, section B.6.
417.6 Analysis
Extraction waters shall be analyzed with the methods listed in annex B, section B.7.
7.7 Normalization
The concentration of analytes present in the extraction water shall be multiplied by calculated normalization factors to account for differences between the actual laboratory evaluation ratio and the weight per volume ratio in table 7.2.
7.7.1 Process media except for activated carbon media and aeration packing media
The concentration reported by the laboratory shall be normalized with the following equation:
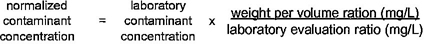
This equation shall be used to normalize filtration media, ion exchange resins, synthetic media, and other media to the weight per volume ratios listed in table 7.2.
7.7.2 Activated carbon media
The concentration reported by the laboratory shall be normalized with the following equation:
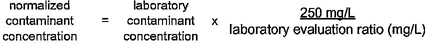
Equation 2 shall be used to normalize activated carbon media (granular or powdered) to a weight per volume ration of 250 mg/L.
7.7.3 Filter precoat media (e.g., perlite, diatomaceous earth)
The concentration reported by the laboratory shall be normalized with the following equation:

Equation 3 shall be used to normalize dosed media (except PAC) to the manufacturer's recommended maximum use concentration.
7.7.4 Aeration packing media
The concentration reported by the laboratory shall be normalized with the following equation:

Where:
SAL = surface area attained during laboratory exposures;
VL = volume of exposure water used during laboratory exposures;
SAF = surface area of the product under field conditions;
VF(flowing) = minimum volume of water the product is exposed to in the field under flowing conditions during a period of time equivalent to the laboratory evaluation.
NOTE – When manufacturer use instructions indicate that the aeration product can be subjected to static conditions in the field, normalized concentrations shall be modified to reflect the static condition. For the static condition, the VF(flowing) parameter shall be substituted with VF(static), which is equal to the volume of water contacting the media under static conditions in the field.
7.8 Evaluation of contaminant concentrations
7.8.1 For process media, normalized contaminant concentrations shall be no greater than their respective SPACs, determined in accordance with annex A.
7.8.2 For aeration packing media which require evaluation to the static condition, the normalized static contaminant concentrations shall be no greater than their respective MCLs or TACs determined in accordance with annex A.
43| Product | Primary use | Analytes |
|---|---|---|
| Activated alumina | Adsorption | Metals1), nickel, and aluminum |
| Aluminum silicates (e.g. zeolites) | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Anthracite | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Diatomaceous earth media | Filtration | Metals1) and radionuclides |
| Garnet | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Granular activated carbon (GAC) | Adsorption | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Gravel | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Ilmenite | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Ion exchange resins | Ion exchange | Residual monomer, other formulation dependent |
| Oxidative media (e.g., manganese green sand) | Oxidation | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Perlite | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Powdered activated carbon (PAC) | Adsorption | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Sand | Filtration | Metals1), GC/MS (base neutral acid scans), and radionuclides |
| Synthetic media | Aeration, filtration | Formulation dependent |
| 1) Metals = Antimony, arsenic, barium, beryllium, cadmium, chromium, copper, lead, mercury, selenium, thallium. | ||
| Media type | Weight per volume1) |
|---|---|
| Adsorption media: Activated alumina GAC and PAC |
625 ± 25 g/L 25 ± 5 g/L |
| Anthracite and gravel2): ≤ 3/8" diameter particles > 3/8" diameter particles |
625 ± 25 g/L 1250 ± 25 g/L |
| Filter precoat media (e.g, perlite, diatomaceous earth) | 10 times the manufacturer's recommended use concentration |
| Filtration media other than anthracite or gravel | 625 ± 25 g/L |
| Ion exchange resins | 625 ± 25 g/L |
| Synthetic media | 625 ± 25 g/L |
| 1) Weight per volume of the product on an “as shipped” basis. 2) For the size range specified, not more than 8% by weight shall be either finer than or coarser than the designated size limit (AWWA B100-96). |
|
| Media type | Maximum laboratory expansion rate of wetted media (by height) (%) |
|---|---|
| Activated alumina | 25 ± 5% |
| Aluminum silicates (zeolites) | 25 ± 5% |
| Anthracite | 25 ± 5% |
| Garnet | 30 ± 5% |
| Granular activated carbon | 30 ± 5% |
| Gravel | 10 ± 5% |
| Ilmenite | 30 ± 5% |
| Manganese greensand | 30 ± 5% |
| Sands | 20 ± 5% |
| Time | Temperature | Comment |
|---|---|---|
| 60 ± 5 minutes | 23 ± 1°C (73 ± 2°F) |
Exposure water is drained/decanted and discarded; the exposure vessel is refilled and exposure is continued. |
| 60 ± 5 minutes | 23 ± 1°C (73 ± 2°F) |
Exposure water is drained/decanted and discarded; the exposure vessel is refilled and exposure is continued. |
| 60 ± 5 minutes | 23 ± 1°C (73 ± 2°F) |
Exposure water is collected and filtered for analyses. |
8.1 Coverage
This section covers devices, components, and materials used therein which are used in treatment/transmission/distribution systems, and are in contact with drinking water intended for human ingestion, drinking water treatment chemicals, or both. Examples are listed in table 8.1. Residential point-of-use and point-of-entry drinking water treatment devices and fire hydrants are not covered by the requirements in this section.
8.2 Definitions
8.2.1 in-line device: any device (used to measure or control the flow of water) installed on a service line or building distribution system downstream of the water main and before endpoint devices.
8.2.2 building distribution system: a continuous system of piping and related fittings, beginning at-the-tap on the main, which is intended to convey potable water to points of usage.
8.3 Device, component, or material requirements
8.3.1 General
Devices, components, or materials shall be considered to have met the requirements of this section if at least one of the following conditions are met:
– the devices, components, or materials covered under this section are tested and evaluated according to the procedures specified in annex B, sections B.4 and B.8; or
– the devices, components, or materials meet the requirements of 8.3.2.
Where all components, materials, or both of a device meet the requirements of this section, the device shall also meet the requirements of this section. Where all materials of a component meet the requirements of this section.
8.3.2 Evaluation of devices, components, or materials tested to other sections of this Standard
For devices, components, or materials, which have been tested to other sections of the standard, the devices, components, or materials shall meet the following criteria:
47– be made of the same alloy(s), composition(s), or formula(s);
– have undergone analogous manufacturing processes;
– have been tested at a temperature that meets or exceeds the required exposure temperature in annex B, section B.4;
– have been conditioned for a period of time not more than 14 days, and exposed for a period of time not less than 12 hours for in-line devices or 24 hours for other mechanical devices; and
– the concentration(s) of the extracted contaminant(s) shall be normalized to the requirements of annex B, section B.8.
8.3.3 Metallic contaminants
When a device or component is qualified through the separate testing of two or more components, the normalized concentrations for each specific metallic contaminant from individual components shall be summed. The total of the normalized metallic contaminant concentrations shall meet the requirements of annex B, section B.8.
48| This table is a generic listing of the types of devices covered in this section of the Standard. This table is not intended to be a complete list of all types of mechanical devices. Inclusion of a product does not indicate either a use endorsement of the product or an automatic acceptance under the provisions of this Standard. | |
| Chemical feeders – Dry feeders (e.g., pellet droppers) |
Switches and sensors (e.g., water level, pressure, temperature, pH) |
| Pressure gas injection systems pumps | |
| Vacuum injection systems | Valves and related fittings (transmission/distribution system) |
| Disinfection/generators – Chlorine dioxide – Hypochlorite – Ozone – Ultraviolet |
Treatment devices used in water treatment facilities (Excludes residential point-of-use and point-of-entry devices) – Aeration technologies – Clarifiers – Electrodialysis technologies – Microfiltration technologies – Mixers – Reverse osmosis technologies – Screens – Strainers – Ultrafiltration technologies |
| Electrical wire (e.g., submersible well pump wire) |
|
| Pupms | |
| In-line devices - building distribution system – Backflow preventers – Building valves – Check valves – Compression fittings – Corporation stops – Curb stops – Expansion tanks – Meter couplings – Meter stops – Pressure regulators – Pressure tanks – Service saddles – Strainers – Valves and fittings – Water meters |
|
| In-line devices specifically excluded – Boiler feed valves – Drilling and tapping machines – Temperature and pressure relief valves – Valves with hose thread outlets – Water meter test benches |
|
9.1 Coverage
This section covers mechanical plumbing devices, components, and materials which are typically installed within the last liter of the distribution system (endpoint devices) and are intended to dispense water for human ingestion. In-line devices are excluded from this section. Point-of-use and point-of-entry water treatment devices are excluded.
9.1.1 Endpoint devices specifically included in the coverage of this section are:
– single-handle and two-handle lavatory faucets (for example: centersets, widespread, mini-spread, and basin cocks), except as exempted in 9.1.2;
– two-hole and single-hole bar faucets;
– single-handle and two-handle kitchen faucets (for example: top mounts, concealed fittings, and wall mounts);
– hot and cold water dispensers;
– drinking fountains, drinking fountain bubblers, and water coolers;
– glass fillers;
– residential refrigerator ice makers;
– supply stops and endpoint control valves; and
– commercial kitchen devices (see 9.2.3), limited to the following:
– pot and kettle fillers (see 9.2.7);
– devices with extended standpipes or rises (see 9.2.5); and
– pre-rinse assemblies which include an auxiliary spout or other outlet.
NOTE 1 – Only the commercial kitchen devices listed above shall be evaluated using the 18.9L (5 gallon) normalization.
NOTE 2 – The bases device to which the pre-rinse component is added shall be considered a commercial kitchen device only if it meets the definition of either a pot and kettle filler (see 9.2.7) or a device with extended standpipes or risers (see 9.2.5).
9.1.2 Endpoint devices specifically exempted from the coverage of this section are:
– bath and shower valves, shower heads of all types, and Roman tub valves;
– all drains;
– backflow prevention devices;
– pre-rinse assemblies which do not include an auxiliary spout or other outlet; and
50– all endpoint devices that are not specifically intended to dispense water for human consumption, including utility, laundry, laboratory, budget, and shampoo fittings: faucets with a hose thread spout end or with a quick disconnect end; faucets that are self-closing, metering, or electronically activated; and nonlavatory hand wash stations.
9.1.3 Endpoint devices which are exempted from the scope of this section shall be permitted to be evaluated at the option of the manufacturer. With the exception of exempted pre-rinse assemblies, all exempted devices shall be evaluated using the 1 L (0.26 gal) draw. Exempted pre-rinse assemblies shall be evaluated using the 18.9 L (5 gal) draw.
9.2 Definitions
9.2.1 cold mix volume adjustment factor (CMV): The cold water volume of the device divided by the total water volume of the device.
9.2.2 cold water volume: The volume of water contained within the portion of the device that is normally contacted by cold water (from inlet to outlet) when the device is connected to hot and cold water supplies under normal operating conditions. The volume excludes the volume of water contained within the portion of the device that is normally contacted only by hot water.
9.2.3 commercial kitchen device: An endpoint device whose sole application is delivery of water for food preparation in commercial kitchens.
9.2.4 endpoint device: Any single device typically installed within the last 1 L of the water distribution system of a building.
9.2.5 extended standpipe or riser device: An endpoint device which includes a vertical component having a minimum height of 16 in (41 cm), measured from the deck to the outlet of the endpoint device, and whose sole application is delivery of water for food preparation in commercial kitchens.
9.2.6 in-line device: Any device, (used to measure or control the flow of water) installed on a service line or building distribution system downstream of the water main and upstream from endpoint devices.
9.2.7 pot and kettle filler: An endpoint device whose sole application is delivery of water to fill pots and kettles in commercial kitchens.
9.2.8 pre-rinse assembly: An endpoint device with a hose and spray whose application is water delivery for the rinsing of tableware in commercial kitchens.
9.2.9 water distribution system (building): A continuous system of piping, devices and related fittings, beginning after the water meter and water meter setting equipment, which is intended to convey potable water in a building to points of usage.
9.3 Device, component, or material requirements
9.3.1 General
Devices, components, or materials shall be considered to have met the requirements of this section if at least one of the following conditions are met:
– The devices, components, or materials covered under this section are tested and evaluated according to procedures specified in annex B, sections B.5 and B.8; or
51– The devices, components, or materials meet the requirements of 9.3.2.
Where all components, materials, or both, of a device meet the requirements of this section, the device shall also meet the requirements of this section. Where all materials of a component meet the requirements of this section, the component shall also meet the requirements of this section.
9.3.2 Evaluation of devices, components, or materials tested to other sections of this standard
For devices, components, or materials which have been tested to other sections of this standard, the devices, components, or materials shall meet the following criteria:
a) be made of the same alloy(s), composition(s), or formula(s);
b) have undergone analogous manufacturing processes;
c) have been tested at a temperature that meets or exceeds the required exposure temperature in annex B, section B.5;
d) have been conditioned for a period of time not more than 19 days and exposed for a period of time not less than 16 hours; and
e) shall have the concentration(s) of the extracted contaminant(s) normalized to the requirements of annex B, section B.8.
9.3.3 Metallic contaminants
When a device or component is qualified through the separate testing of two or more components, the normalized concentrations for each specific metallic contaminant from individual components shall be summed. The total of the normalized metallic contaminant concentrations shall meet the requirements of section 9.5.
9.4 Exposure and normalization
Samples for testing shall be prepared and exposed, and the extractant water analyzed as required in annex B, section B.5. The number of samples tested shall be determined as outlined in annex B, section B.5.
Exposure of endpoint samples, except for hot water dispenser samples, shall be performed at 23 ± 2EC (73 ± 4EF).
The concentration of extracted contaminants shall be normalized to end use conditions according to the normalization procedure outlined in annex B, section B.8 for endpoint devices, components, and materials. All endpoint devices, components, or materials, other than commercial kitchen devices, shall be evaluated using the highest surface area-to-volume product as the test sample, and shall be normalized using the 1 L (0.26 gal) first draw. Commercial kitchen devices shall be evaluated using the highest surface area-to-volume product as the test sample and shall be normalized using the 18.9 L (5 gal) first draw.
9.5 Evaluation of normalized contaminant concentrations
9.5.1 Evaluation of lead
For endpoint devices other than commercial kitchen devices, the lead test statistic Q shall not exceed 11 µg when normalized for the 1 L (0.26 gal) first draw sample. For commercial kitchen devices, the lead test statistic Q shall not exceed 11 µg when normalized for the 18.9 L (5 gal) first draw sample.
52NOTE – The limit of 11 µg for lead is based on a limit of 15 µg total lead, including lead contributed from the device interior as well as from sources other than the device, which is assumed to be 4 µg.
9.5.2 Evaluation of non-lead contaminants
For endpoint devices other than commercial kitchen devices, the normalized concentration of a nonlead contaminant shall not exceed its SPAC (calculated in accordance with annex A) when normalized for the 1 L (0.26 gal) first draw sample. For commercial kitchen devices, the normalized concentration of a nonlead contaminant shall not exceed its SPAC when normalized for the 18.9 L (5 gal) first draw sample.
53 54This annex defines the toxicological review and evaluation procedures for the evaluation of substances imparted to drinking water through contact with drinking water system components. It is intended to establish the human health risk, if any, of the substances imparted to drinking water under the anticipated use conditions of the product. Annex D (normative) and annex E (informative) of this Standard contain evaluation criteria that have been determined according to the requirements of this annex.
The following general procedure shall be used to evaluate drinking water substances under this Standard:
a) A determination shall be made as to whether a published (publically available in printed or electronic format) and peer reviewed quantitative risk assessment for the substance is available.
b) When a quantitative risk assessment is available, the reviewer shall determine whether the assessment is currently used in the promulgation of a drinking water regulation or published health advisory for the substance (see the requirements of annex A, section A.3):
– If the assessment is used in the promulgation of a drinking water regulation, the Single Product Allowable Concentration (SPAC) shall be derived from the regulatory value(s); or
– If the assessment is not the basis of a drinking water regulation, the assessment and its corresponding reference dose shall be reviewed for its appropriateness in evaluating the human health risk of the drinking water substance.
NOTE – When reviewing an assessment used in the promulgation of a drinking water regulation, it is recommended that the regulatory authority be contacted to verify the currency of the assessment under consideration.
c) If a published and peer reviewed quantitative risk assessment is not currently available for the substance, the Total Allowable Concentration (TAC) and SPAC shall be derived after review of the available toxicology data for the substance (see annex A, section A.4).
– When the data requirements for quantitative risk assessment are satisfied (see annex A, section A.4.2 and table A1), a qualitative risk assessment shall be performed according to annex A, section A.7; or
– When the data requirements for quantitative risk assessment are satisfied (see annex A, section A.4.3 and table A2), a quantitative risk assessment shall be performed according to annex A, section A.7.
Figure A1, annex A, provides an overview of the toxicity data review requirements of this annex.
A.2.1 benchmark dose: The lower 95% confidence limit on the dose that would be expected to produce a specified response in X% of a test population. This dose may be expressed as BMDx (adapted from Barnes et al., 1995).
A1NOTE – For the purposes of this standard, the benchmark dose shall be calculated at the 10% response level.
A.2.2 continuous data: A measurement of effect that is expressed on a continuous scale e.g., body weight or serum enzyme levels (USEPA, 1995).
A.2.3 critical effect: The first adverse effect, or its known precursor, that occurs as the dose rate increases (USEPA, 1994).
A.2.4 ED10: Effective dose 10; a dose estimated to cause a 10% response in a test population (USEPA, 1996a).
A.2.5 genetic toxicity: Direct interaction with DNA that has the potential to cause heritable changes to the cell.
A.2.6 health hazards (types of) (USEPA, 1994 and 1999):
A.2.6.1 acute toxicity: Effects which occur immediately or develop rapidly after a single administration of a substance. Acute toxicity may also be referred to as immediate toxicity.
A.2.6.2 allergic reaction: Adverse reaction to a chemical resulting from previous sensitization to that chemical or to a structurally similar one.
A.2.6.3 chronic effect: An effect which occurs as a result of repeated or long term (chronic) exposures.
A.2.6.4 chronic exposure: Multiple exposures occurring over an extended period of time, or a significant fraction of the animal's or the individual's lifetime.
A.2.6.5 chronic toxicity: The capability of a substance to cause adverse human health effects as a result of chronic exposure.
A.2.6.6 irreversible toxicity: Toxic effects to a tissue which cannot be repaired.
A.2.6.7 local toxicity: Effects which occur at the site of first contact between the biological system and the toxicant.
A.2.6.8 reversible toxicity: Toxic effects which can be repaired, usually by a specific tissue's ability to regenerate or mend itself after chemical exposure.
A.2.6.9 systemic toxicity: Effects that are elicited after absorption and distribution of a toxicant from its entry point to its target tissue.
A.2.7 LED10: Lowest effective dose 10; the lower 95% confidence limit on a dose estimated to cause a 10% response in a test population (USEPA, 1996a).
A.2.8 lowest observed adverse effect level (LOAEL): The lowest exposure concentration at which statistically or biologically significant increases in frequency or severity of effects are observed between the exposed population and its appropriate control group (USEPA, 1994).
A.2.9 margin of exposure (MOE): The LED10 or other point of departure, such as a NOAEL, divided by the environmental dose of interest (USEPA, 1996a).
A.2.10 model: A mathematical function with parameters which can be adjusted so that the function closely describes a set of empirical data. A mathematical or mechanistic model is usually based on biological or physical mechanisms, and has model parameters that have real world interpretation. Statistical or empirical models are curve-fitted to data where the math function used is selected for its numerical properties and accuracy. Extrapolation from mechanistic models (e.g., pharmacokinetic equations) usually carries higher confidence than extrapolation using empirical models (e.g., logit) (USEPA, 1994).
A2A.2.11 no observed adverse effect level (NOAEL): An exposure concentration at which no statistically or biologically significant increases in the frequency or severity of adverse effects are observed between an exposed population and its appropriate control. Some physiological effects may be produced at this concentration, but they are not considered as toxicologically significant or adverse, or as precursors to adverse effects (USEPA, 1994).
A.2.12 non-regulated substance: A substance for which a statutory concentration limit does not exist.
A.2.13 peer review: A documented critical review of a scientific or technical work product conducted by qualified individuals or organizations who are independent of those who performed the work, but who are collectively equivalent or superior in technical expertise to those who performed the work. It includes an in-depth assessment of the assumptions, calculations, extrapolations, alternate interpretations, methodology, acceptance criteria, and conclusions pertaining to the work product and the documentation that supports the conclusions reached in the report. Peer review is intended to ensure that the work product is technically adequate, competently performed, properly documented, and satisfies established requirements (USEPA, 1998).
A.2.14 point of departure: A date point or an estimated point that can be considered to be in the range of observation. The standard point of departure is the LED10, which is the lower 95% confidence limit on a dose associated with 10% extra risk (adapted from Barnes et al., 1995).
A.2.15 qualitative risk assessment: An estimation of the risk associated with the exposure to a substance using a non-quantitative methodology.
A.2.16 quantal data: A dichotomous measure of effect; each animal is scored “normal” or “affected” and the measure of effect is the proportion of scored animals that are affected (USEPA, 1995).
A.2.17 quantitative risk assessment: An estimation of the risk associated with the exposure to a substance using a methodology which employs evaluation of dose response relationships.
A.2.18 range of extrapolation: Doses which are outside of the range of empirical observation in animal studies, human studies, or both (adapted from Barnes et al., 1995).
A.2.19 range of observation: Doses which are within the range of empirical observation in animal studies, human studies, or both (adapted from Barnes et al., 1995).
A.2.20 reference dose (RfD): An estimate (with uncertainty spanning approximately an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime (USEPA, 1994).
A.2.21 regulated substance: A substance for which a quantitative human health risk assessment has been performed and utilized in promulgation of a statutory concentration limit for drinking water.
A.2.22 toxicodynamics: Variations in the inherent sensitivity of a species or individual to chemical-induced toxicity, resulting from differences in host factors that influence the toxic response of a target organ to a specified dose (TERA, 1996).
A.2.23 toxicokinetics: Variations in absorption, distribution, metabolism, and excretion of a compound which account for differences in the amount of parent compound or active metabolite(s) available to a target organ (TERA, 1996).
A.2.24 treatment technique: A technology or one or more procedures used to control the concentration of a substance in a drinking water supply when it is neither technically nor economically feasible to ascertain the concentration of the substance (U.S. Safe Drinking Water Act, 1996).
A3A.2.25 weight-of-evidence: The extent to which the available biomedical data support the hypothesis that a substance causes cancer or other toxic effects in humans (adapted from USEPA, 1994).
A.3.1 General requirements
Evaluation of all published risk assessments shall include review of the written risk assessment document and a determination of whether additional toxicity data exist which were not considered in the assessment. If additional toxicity data are identified which were not considered in the risk assessment, the risk assessment shall be updated in accordance with annex A, section A.4.
The following shall be documented when utilizing an existing risk assessment:
– the source of the risk assessment;
– identification and discussion of any data not addressed by the assessment; and
– comparison and contrast of the existing risk assessment to the requirements of annex A, section A.4 with respect to selection of uncertainty factors or other assumptions.
A.3.2 Substances regulated by USEPA or Health Canada
If a substance is regulated under the USEPA's National Primary Drinking Water Regulations and EPA has finalized a Maximum Contaminant Level (MCL) or other means of regulation such as a treatment technique (see annex A, section A.2.18), no additional collection of toxicological data shall be required prior to performance of the risk estimation (see annex A, section A.6.1). Where Health Canada has finalized a Maximum Allowable Concentration (MAC), no additional toxicological evaluation shall be required prior to performance of the risk estimation (see annex A, section A.6.1). Annex D contains a list of regulatory values (MCL OR MAC) and their corresponding SPACs. This list includes consensus evaluation criteria for those substances which are regulated by both countries.
A.3.2 Substances regulated by other agencies
If a substance is regulated by agencies including the U.S. Food and Drug Administration (Code of Federal Regulations, Title 21 Food and Drug Regulations), or state, national, or international regulatory bodies other than those specified in annex A, section A.3.2, the relevance of the regulation to drinking water shall be evaluated. This evaluation shall include a review of the quantitative risk assessment which supports the regulation, and a determination of whether additional toxicity data exist which have not been considered in the current assessment. No additional collection of toxicological data shall be required when the regulation provides sufficient information for performance of the risk estimation (see annex A, section A.6.1). If additional toxicity data are identified which were not considered in the current risk assessment, a revised risk assessment incorporating those data shall be performed as indicated in annex A, sections A.4 and A.7.
A.3.4 Evaluation of multiple published risk assessments
When multiple published assessments are available for a specific substance, the available assessments shall be reviewed and a rationale shall be provided for the selection of the assessment considered to be the most appropriate for the evaluation of human exposure through drinking water. Factors used to determine the appropriate assessment shall include, but not be limited to the following:
– completeness and currency of the data review of each assessment;
– technical competence of the organization(s) which sponsored the assessment; and
A4– species and route(s) of exposure for which the assessment was performed.
When multiple published risk assessments are reviewed and are determined to be of equivalent quality, the following hierarchy shall be used to select the appropriate assessment, based on sponsoring organization:
– USEPA;
– Health Canada;
– international bodies such as the World Health Organization (WHO) or the International Programme on Chemical Safety (IPCS);
– European bodies such as the Drinking Water Inspectorate (DWI) and KIWA;
– entities such as other federal or state regulatory agencies, private corporations, industry associations, or individuals,
A.4.1 General requirements
For each substance requiring a new or updated risk assessment, toxicity data to be considered shall include, but not be limited to, assays of genetic toxicity, acute toxicity (1 to 14 day exposure), short-term toxicity (14 to 28 day exposure), subchronic toxicity (90 day exposure), reproductive toxicity, developmental toxicity, immunotoxicity, neurotoxicity, chronic toxicity (including carcinogenicity), and human data (clinical, epidemiological, or occupational) when available. To more fully understand the toxic potential of the substance, supplemental studies shall be reviewed, including, but not limited to mode or machanism of action, pharmacokinetics, pharmacodynamics, sensitization, endocrine disruption, and other endpoints, as well as studies using routes of exposure other than ingestion. Structure activity relationships, physical and chemical properties, and any other chemical specific information relevant to the risk assessment shall also be reviewed.
Toxicity testing shall be performed in accordance with the most recent adopted toxicity testing protocols such as those described by the Organization For Economic Cooperation and Development (OECD), U.S. Environmental Protection Agency (USEPA), and U.S. Food and Drug Administration (FDA). All studies shall be reviewed for compliance with Good Laboratory Practice (21 CFR, Pt 5840 CFR, Pt 792).
NOTE – Review of the study according to the approach suggested in Klimisch et. al, 1997 may also be used to determine the quality of reported data.
A weight-of-evidence approach shall be employed in evaluating the results of the available toxicity data. This approach shall include considering the likelihood of hazard to human health and the conditions under which such hazard may be expressed. A characterization of the expression of such effects shall also be included, as well as the consideration of the substance's apparent mode of action. The quality and quantity of toxicity data available for the substance shall determine whether the evaluation is performed using a qualitative risk assessment approach (see annex A, section A.4.2) or a quantitative risk assessment approach (see annex A, section A.4.3).
A.4.2 Data requirements for qualitative risk assessment
Toxicity testing requirements for the qualitative risk assessment procedure are defined in annex A, table A.1.A minimum data set consisting of a gene mutation assay and a chromosomal aberration assay shall be required for the performance of a qualitative risk assessment. Modifications in the specified toxicity testing requirements (inclusions or exclusions) shall be permitted when well-supported by peer reviewed scientific judgement and rationale.
A5NOTE – Modifications may include, but are not limited to the following types of considerations: alternate assays of genetic toxicity, and supplemental toxicity studies other than those specified.
Required studies and available supplemental studies shall be reviewed in order to perform a qualitative risk estimation in accordance with annex A, section A.7.2.
A.4.3 Data requirements for quantitative risk assessment
Toxicity testing requirements for the quantitative risk assessment procedure are defined in annex A, table A2. A minimum data set consisting of a gene mutation assay, a chromosomal aberration assay, and a subchronic toxicity study shall be required for the performance of a quantitative risk assessment. The required studies and preferred criteria are defined in annex A, table A2. Modifications to the minimum data set shall be permitted when well-supported by peer reviewed scientific judgement and rationale.
NOTE – Modifications may include, but are not limited to acceptance of studies using alternate routes of exposure, alternate assays of genetic toxicity, and supplemental toxicity studies other than those specified.
Required studies, additional studies, and available supplemental studies shall be reviewed in order to perform a quantitative risk estimation in accordance with annex A, section A.7.3.
Additional studies for the evaluation of reproductive and developmental toxicity (as specified in annex A, table A2) shall be required to be reviewed when:
– results of the required minimum data set studies and any supplemental studies indicated toxicity to the reproductive or endocrine tissues of one or both sexes of experimental animals; or
– the compound under evaluation is closely related to a known reproductive or developmental toxicant.
Extractants from products used in contact with drinking water may be elevated initially, but rapidly decline with continued product contact with water. Examples include, but are not limited to, solvent-containing coatings and solvent cements. Short-term exposure paradigms, appropriate for potentially high initial substance concentrations, shall be used to evaluate potential acute risk to human health of short-term exposures. The short-term exposure period shall be defined as the first 14 days of in-service life of the product.
Sound scientific judgement shall be used to determine whether calculation of a Short-Term Exposure Level (STEL) is appropriate for a given contaminant. The NOAEL OR LOAEL for the critical short-term hazard of the substance shall be identified. The following types of studies shall be considered for identification of short-term hazard:
– short-term (less than 90 days duration) toxicity study in rodents or other appropriate species with a minimum 14-day post-treatment observation period, clinical observations, hematology and clinical chemistry, and gross pathology (preferably an oral study in rodents);
– reproduction or developmental assays (for substances having these endpoints as the critical effects); or
– subchronic 90-day study in rodents or other species (preferably an oral study in rats).
The critical study shall be used to calculate a Short-Term Exposure Level (STEL) in accordance with annex A, section A.8.
Selection of uncertainty factors for calculation of a STEL shall consider the quality and completeness of the database for assessing potential short-effects. Selection of uncertainty factors shall also consider data which
A6quantify interspecies and interspecies variations. Other parameters that shall be considered in the determination of a STEL include identification of any sensitive subpopulations, the potential for adverse taste and odor, and solubility limitations at the calculated STEL. The STEL shall be calculated using assumptions to protect for a child's exposure to the contaminant in the absence of data that demonstrate adults are more sensitive than children. In the absence of appropriate data to calculate a STEL, see annex A, section A.7.1.2.
Calculation of the SPAC is intended to account for the potential contribution of a single substance by multiple products or materials in the drinking water treatment and distribution system. In any given drinking water treatment and distribution system, a variety of products and materials may be added to or contact the treated water prior to ingestion. The SPAC calculation is intended to ensure that the total contribution of a single substance from all potential sources in the drinking water treatment and distribution system does not exceed its acceptable concentration.
A.6.1 SPAC calculation for regulated substances
To calculate the SPAC, an estimate of the number of potential sources of the substance from all products in the drinking water treatment and distribution system shall be determined. The SPAC shall be calculated as follows:

In the absence of specific data regarding the number of potential sources of the substance in the drinking water treatment and distribution system, the SPAC shall be calculated as 10% of the promulgated regulatory value.
A.6.2 SPAC calculation for other published risk assessments
Review of the risk assessment shall be include evaluation of the risk estimation, if one is provided. If the existing risk estimation has been performed in a manner consistent with the procedures in annex A, section A.7.3 for non-carcinogenic or carcinogenic endpoints, the SPAC shall be calculated as follows:

In the absence of specific data regarding the number of potential sources of the substance in the drinking water treatment and distribution system, the SPAC shall be calculated as 10% of the existing risk estimation.
If the existing risk estimation is not consistent with annex A, section A.7.3, or a risk estimation is not provided, a TAC and SPAC shall be calculated for the substance according to the procedures in annex A, section A.7.3.
The method of risk estimation used for new and updated risk assessments shall be determined by the quantity and quality of toxicity data identified for the contaminant of concern (see annex A, section A.4). When available toxicity data are insufficient to perform the qualitative or quantitative risk assessments, or when toxicity data are available, but the normalized contaminant concentration does not exceed the applicable threshold of evaluation value, a threshold of evaluation shall be determined for the substance according to annex A, section A.7.1 if applicable. For all other data sets, the risk estimation shall be performed according to annex A, sections A.7.2 or A.7.3.
A7A.7.1 Threshold of evaluation
The following thresholds of evaluation shall be considered when available toxicity data do not meet the minimum requirements to perform a risk estimation using either the qualitative or quantitative approaches. Application of the threshold of evaluation shall also be considered for the evaluation of normalized contaminant concentrations which do not have existing risk assessments, and which do not exceed the defined threshold of evaluation concentrations. In this case, a qualitative review of the available data shall be performed to determine whether adverse health effects can result at the threshold of evaluation exposure concentrations defined in annex A, section A.7.1.1.
A.7.1.1 Threshold of evaluation for chronic exposure
Performance of a risk assessment shall not be required for an individual substance having a normalized concentration less than or equal to the following threshold of evaluation values:
– static normalization conditions:
– toxicity testing shall not be required for an individual substance having a normalized concentration less than or equal to the threshold of evaluation value of 3µg/L.
– flowing normalization conditions:
– toxicity testing shall not be required for an individual substance having a normalized concentration less than or equal to the threshold of evaluation value of 0.3 µg/L.
These threshold of evaluation values shall not apply to any substance for which available toxicity data and sound scientific judgement such as structure activity relationships indicate that an adverse health effect results at these exposure concentrations.
A.7.1.2 Threshold of evaluation for short-term exposure
If an appropriate short-term toxic effect is not identified by the available data, the initial (Day 1) laboratory concentration shall not exceed 10 µg/L. This threshold of evaluation value shall not apply to any chemical for which available toxicity data and sound scientific judgement such as structure activity relationships indicate that an adverse health effect can result at the 10 µg/L concentration upon short-term exposure to the chemical.
A.7.2 TAC determination for qualitative risk assessment
TACs for qualitative risk assessments shall be determined as indicated in annex A, table A3.
A.7.3 TAC calculation for quantitative risk assessment
The procedure used to calculate the TAC for a new risk assessment (including qualitative assessments that are updated upon generation of new data) shall be determined by the toxicologic endpoint identified as the critical effect (see annex A, section A.2.3). For a substance having a non-carcinogenic endpoint, a TAC shall be calculated according to annex A, section A.7.3.1. For a substance having carcinogenic potential, a TAC shall be calculated according to annex A, section A.7.3.2.
The minimum data set for the quantitative risk assessment (as defined in annex A, section A.4.3 and table A2) shall first be evaluated for genotoxic potential according to the requirements of annex A, table A3. Based on the review of genotoxic potential, the need for supplemental studies or chronic toxicity and carcinogenesis data shall be determined.
A8A.7.3.1 Assessment of noncarcinogenic endpoints
For noncarcinogenic endpoints, the TAC shall be calculated using either the NOAEL/LOAEL procedure outlined in annex A, section A.7.3.1.1, or the benchmark dose (BMD) procedure outlined in annex A, section A.7.3.1.2, as appropriate. The rationale for the selection of the procedure shall be provided in the assessment.
NOTE – Selection of the appropriate TAC calculation procedure will depend on the characteristics of the data set identified for the substance. Simple data sets consisting of a small number of studies may be best evaluated using the procedure in annex A, section A.7.3.1.1. Complex data sets consisting of several studies, or which involve reproduction or developmental endpoints may be best evaluated using the benchmark dose procedure in annex A, section A.7.3.1.2. The appropriateness of the fit of the data to the BMD shall also be considered.
A.7.3.1.1 NOAEL or LOAEL approach
The substance data set shall be reviewed in its entirety, and the highest NOAEL for the most appropriate test species, relevant route of exposure, study duration, mechanism, tissue response, and toxicological endpoint shall be identified. If a NOAEL cannot be clearly defined from the data, the lowest LOAEL for the most appropriate test species, relevant route of exposure, and toxicological endpoint shall be utilized.
The general procedure for calculating the TAC using this approach is as follows:
a) determine the critical study and effect from which the NOAEL or LOAEL will be identified according to the following hierarchy (USEPA, 1993 and Dourson et al., 1994):
– adequate studies in humans;
– adequate studies in animal models most biologically relevant to humans (e.g., primates), or that demonstrate similar pharmacokinetics to humans;
– adequate studies in the most sensitive animal species (the species showing an adverse effect at the lowest administered dose using an appropriate vehicle, an adequate study duration, and a relevant route of exposure); and
– effects that are biologically-relevant to humans.
b) calculate the reference dose (RfD) according to the following equation (based on USEPA, 1993):
NOTE – When other than daily dosing was used in the critical study, the RfD calculation shall be adjusted to reflect a daily dosing schedule.
c) calculate the TAC based on the RfD with adjustment for significant contribution(s) of the substance from sources other than drinking water according to the following equation:
Where:
– NOAEL = Highest NOAEL for the critical effect in the most appropriate species identified after review of data set; if a NOAEL is not defined, the LOAEL shall be used with a corresponding adjustment in the uncertainty factor (see annex A, table A4).
– BW = Assumed body weight of individual to be protected in kg (generally 10 kg for a child,,
A9and 70 kg for an adult).
– UF = Uncertainty factor (total) based upon the applicability of the test data in extrapolating to actual conditions of human exposure (see annex A, table A4). These are often referred to as safety factors.
– DWI = Drinking Water Intake is the assumed average daily drinking water consumption per day (generally 1 L for a child and 2 L for an adult).
NOTE 1 – In the absence of data to determine the drinking water contribution of a substance, a default drinking water contribution of 20% shall be applied (USEPA, 1991).
NOTE 2 – If calculation of the non-drinking water contribution of a substance exceeds the value of the (RfD × BW), verify that all potential exposures to the substance in the critical study have been accounted, e.g., is the substance present as a contaminant in the feed as well as dosed into the drinking water, etc.
A.7.3.1.2 Benchmark dose approach
The benchmark dose level (BMDL) for the substance shall be calculated by modeling the substance's dose response curve for the critical effect in the region of observed responses. The benchmark response (BMR) concentration shall be determined by whether the critical response is a continuous endpoint measurement or a quantal endpoint measurement. The BMR shall be calculated at the 10% response level.
The general procedure for calculating the TAC using the BMDL is as follows:
a) calculate the reference dose (RfD) according to the following equation:
NOTE – When other than daily dosing was used in the critical study, the RfD calculation shall be adjusted to reflect a daily dosing schedule.
b) calculate the TAC based on the RfD with adjustment for significant contribution(s) of the substance from sources other than water according to the following equation:
Where:
– BMDL = The lower confidence limit on the dose that produces a specified magnitude of change (10%) in a specified adverse response (BMD10).
– BW = Assumed body weight of individual to be protected in kg (generally 10 kg for a child, and 70 kg for an adult).
– UF = Uncertainty factor (total) based upon the applicability of the test data in extrapolating to actual conditions of human exposure (see annex A, table A4). These are often referred to as safety factors.
– DWI = Drinking Water Intake is the assumed average daily drinking water consumption per day (generally 1 L for a child and 2 L for an adult).
NOTE 1 – In the absence of data to determine the drinking water contribution of a substance, a
A10default drinking water contribution of 20% shall be applied (USEPA, 1991).
NOTE 2 – If calculation of the non-drinking water contribution of a substance exceeds the value of the (RfD × BW), verify that all potential exposures to the substance in the critical study have been accounted, e.g., is the substance present as a contaminant in the feed as well as dosed into the drinking water, etc.
A.7.3.1.3 Selection of uncertainty factors (UF)
Uncertainty factors used for the risk estimation shall include consideration of the areas of uncertainty listed in annex A, table A4. A default value of 10 shall be used for individual areas of uncertainty when adequate data are not available to support a data-derived uncertainty factor. Selection of the values of each uncertainty factor shall consider the following criteria (adapted from Dourson et al., 19961)).
A.7.3.1.3.1 Human variability
Selection of the human variability factor shall be based on the availability of data that identify sensitive subpopulations of humans. If sufficient data are available to quantitate the toxicokinetic and toxicodynamic variability of humans (see annex A, sections A.2.22 and A.2.23), factor values of 3, 1, or a value determined from the data shall be considered. In the absence of these data, the default value of 10 shall be used.
A.7.3.1.3.2 Interspecies variability
Selection of the interspecies variability factor shall be based on the availability of data that allow for a quantitative extrapolation of animal dose to the equivalent human dose for effects of similar magnitude or for a NOAEL. This includes scientifically documented differences or similarities in physiology, metabolism and toxic response(s) between experimental animals and humans. If sufficient data are available to quantitate the toxicokinetic and toxicodynamic variabilities between experimental animals and humans (see annex A, sections A.2.22 and A.2.23), factor values of 3, 1, or a value determined from the data shall be considered. In the absence of these data, the default value of 10 shall be used.
A.7.3.1.3.3 Subchronic to chronic extrapolation
Selection of the factor for subchronic to chronic extrapolation shall be based on the availability of data that allow for quantitative extrapolation of the critical effect after subchronic exposure to that after chronic exposure. Selection shall also consider whether NOAELs differ quantitatively when different critical effects are observed after subchronic and chronic exposure to the compound. When the critical effect is identified from a study of chronic exposure, the factor value shall be 1. When sufficient data are available to quantitate the difference in the critical effect after subchronic and chronic exposure, or when the principal studies do not suggest that duration of exposure is a determinant of the critical effects, a factor value of 3 or a value determined from the data shall be considered. In the absence of these data, the default value of 10 shall be used.
1)The Food Quality Protection Act (FQPA) of 1996 re-remphasized the review and evaluation of toxicity data for the protection of children's health. USEPA has been very responsive to this initiative and published a draft document outlining the use of an uncertainty factor for children's protection and other database deficiencies (USEPA, 1999). Currently this factor is applied to pesticide evaluations only. In addition, publications by Renwick (1993) and the International Programme for Chemical Safety (IPCS) (1994) suggest the use of specific data in lieu of default values for uncertainty factors. This suggestion has been actively discussed at subsequent IPCS meetings and several individual chemical examples have been published (IPCS, 1999). The use of data-derived uncertainty factors, or judgement, as replacements to default values of 10-fold for each area of uncertainty is encouraged by several federal and international agencies and organizations (Meek, 1994; Dourson, 1994).
A11A.7.3.1.3.4 Database sufficiency
Selection of the factor for database sufficiency shall be based on the ability of the existing data to support a scientific judgement of the likely critical effect of exposure to the compound. When data exist from a minimum of five core studies (two chronic bioassays in different species, one two-generation reproductive study, and two developmental toxicity studies in different species), a factor value of 1 shall be considered. When several, but not all, of the core studies are available, a factor value of 3 shall be considered. When several of the core studies are unavailable, the default value of 10 shall be used.
A.7.3.1.3.5 LOAEL to NOAEL extrapolation
Selection of the factor for LOAEL to NOAEL extrapolation shall be based on the ability of the existing data to allow the use of a LOAEL rather than a NOAEL for non-cancer risk estimation. If a well-defined NOAEL is identified, the factor value shall be 1. When the identified LOAEL is for a minimally adverse or reversible toxic effect, a factor value of 3 shall be considered. When the identified LOAEL is for a severe or irreversible toxic effect, a factor value of 10 shall be used.
A.7.3.2 Assessment of carcinogenic endpoints
Risk assessment for carcinogenic endpoints shall be performed using the linear approach, the non-linear approach, or both, consistent with the proposed USEPA Cancer Risk Assessment Guidelines (USEPA, 1996a). For substances which have been identified as known or likely human carcinogens (as defined by these Guidelines), a dose response assessment shall be performed. This dose response assessment shall include analysis of dose both in the range of observation (animal and human studies) and in the range of extrapolation to lower doses.
A.7.3.2.1 Analysis in the range of observation
Curve-fitting models shall be selected based on the characteristics of the response data in the observed range. The model shall be selected, to the extent possible, based on the biological mode of action of the substance taken together in a weight of evidence evaluation of the available toxicological and biological data. The selected model shall be used to determine the LED10, which will either be the point of departure (see annex A, section A.2.14) for linear low dose extrapolation or the basis of the margin of exposure (MOE) analysis (see annex A, section A.2.9) for a non-linear assessment.
NOTE – See annex A, figure A2 for a graphical representation of this analysis.
The following types of models shall be considered, as appropriate to the mode of action of the substance under evaluation, the availability of adequate data, and the current state of risk assessment approaches:
– statistical or distribution models;
– log-probit
– logit
– Weibull– mechanistic models;
– one-hit
– multihit
– multistage
– cell kinetic multistage– model enhancement and dose scaling;
– time to tumor response
– physiologically based toxicokinetic models A12
– biologically based dose-response models
– surface area conversion
If none of the available models provide a reasonable fit to the dataset, the following shall be considered to see if lack of fit can be resolved (USEPA, 1995):
– interference at higher dose concentrations from competing mechanisms of toxicity that are a progressive form of the response of interest;
– saturation of metabolic or delivery systems for the ultimate toxicant at higher dose concentrations; and
– interference at higher dose concentrations due to toxic effects unrelated to the response of interest.
NOTE – When adjusting for these possibilities does not provide a reasonable fit, one suggested approach is to delete the high dose data and refit the models based on the lower dose concentrations since these doses are the most informative of the exposure concentrations anticipated to be encountered by humans.
A.7.3.2.2 Analysis in the range of extrapolation
The choice of procedure for low dose extrapolation shall be based on the biological mode of action of the substance. Depending upon the quantity and quality of the data, and upon the conclusion of the weight of evidence evaluation, the following procedures shall be used: linear, non-linear, or linear and non-linear.
A.7.3.2.2.1 Linear analysis
The linear default assumption shall be used when the toxicological data support a mode of action due to DNA reactivity or another mode of action which is anticipated to be linear in nature. It shall also be used when no data are available to justify an alternate approach. For linear extrapolation, a straight line is constructed from the point of departure on the dose response curve to the zero dose/zero response point.
A.7.3.2.2.2 Non-linear analysis
The non-linear default assumption shall be used when the toxicological data are sufficient to support the assumption of a non-linear mechanism of action, and no evidence for linearity is available. A margin of exposure (MOE) analysis shall be used for non-linear assessment. The margin of exposure shall be calculated by dividing the point of departure by the human exposure concentration of interest.
A.7.3.2.2.3 Linear and non-linear analysis
Linear and non-linear assessments shall be provided when the weight of evidence or the mode of action analysis indicates differing modes of action for different target tissues, or to evaluate the implications of complex dose response relationships. Where the results of linear and non-linear evaluations differ, the range of estimates shall be discussed, along with a justification for the estimate used in evaluation of the substance.
A.7.3.3 Determination of the TAC for carcinogenic endpoints
The selected model shall be used to determine the dose equivalent to the LED10. For linear analyses, the TAC shall be determined by linear extrapolation of the LED10 to the origin of the dose response curve for the selected level of risk. For non-linear analyses, the TAC shall be equal to the human exposure concentration of interest which represents the selected MOE (LED10/exposure of interest). For both types of analyses, the level of risk or margin of exposure shall be selected in accordance with the USEPA Cancer Risk Assessment Guidelines (USEPA, 1996a).
A13A.7.4 Single Product Allowable Concentration (SPAC) calculation for new or updated risk assessments
Calculation of the SPAC is intended to account for potential contribution of a single substance by multiple products or materials in the drinking water treatment and distribution system. In any given drinking water treatment and distribution system, a variety of products and materials may be added to or contact the treated water prior to ingestion. The SPAC calculation is intended to ensure that the total contribution of a single substance from all potential sources in the drinking water treatment and distribution system does not exceed its acceptable concentration.
A.7.4.1 SPAC determination for qualitative risk assessment
The SPAC for qualitative risk assessments shall be equal to the value of the TAC.
A.7.4.2 SPAC determination for quantitative risk assessment
To calculate the SPAC, an estimate of the number of potential sources of the substance from all products in the drinking water treatment and distribution system shall be determined. The SPAC shall be calculated as follows:
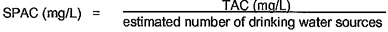
In the absence of specific data regarding the number of potential sources of the substance in the drinking water treatment and distribution system, the SPAC shall be calculated as 10% of the TAC.
The STEL shall be calculated using the following equation:
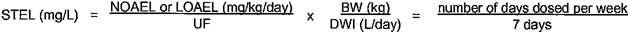
A14NOTE – When other than daily dosing was used in the critical study, the STEL calculation shall be adjusted to reflect the closing schedule.
Where:
– NOAEL = Highest NOAEL for the critical effect in a study of less than or equal to 90 days duration (see annex A, section A.5); if a NOAEL is not defined, the LOAEL shall be used with a corresponding adjustment to the uncertainty factor (see annex A, table A4).
– BW = Assumed body weight of the individual to be protected (in kg), generally 10 kg for a child and 70 kg for an adult. The default body weight shall reflect that of a child, in the absence of data which demonstrate that adults are more sensitive than children.
– UF = Uncertainty factor based upon the applicability of the test data in extrapolating to actual conditions of human exposure (see annex A, table A4); also referred to as safety factors.
– DWI = Drinking Water Intake is the assumed average daily drinking water consumption in L/day, generally 1 L for a child and 2 L for an adult. The default water consumption shall reflect that of a child, in the absence of data which demonstrate that adults are more sensitive than children.
A.9.1 Identification of the need for chemical class-based evaluation criteria
Annex A provides a threshold of evaluation to be utilized when the required toxicity data to perform qualitative or quantitative risk assessment (see annex A, section A.4) are unavailable, or when the required data are available, but the normalized contaminant concentrations do not exceed the threshold of evaluation concentrations (see annex A, section A.7.1). However, normalized contaminant concentrations for chemicals which do not meet minimum data requirements may exceed the threshold of evaluation concentrations. In this case it may be possible to determine chemical class-based evaluation criteria for the substance on the basis of the known toxicities of other chemicals of similar structure and functionality. Those criteria can then be used as surrogates to the TAC and SPAC established on the basis of chemical-specific information.
Class-based evaluation criteria shall not be used for any substance for which adequate data exist to perform a chemical-specific risk assessment.
A.9.2 Procedure for defining class-based evaluation criteria
A.9.2.1 Establishment of the chemical class
The chemical class for which the class-based evaluation criteria are to be established shall consist of a clearly defined and closely related group of substances, and shall be defined according to chemical structure (e.g., aliphatic, aromatic, etc.), primary chemical functional group(s) (e.g., alcohol, aldehyde, ketone, etc.), and molecular weight or weight range.
A.9.2.2 Review of chemical class toxicity information
Once the chemical class has been defined according to annex A, section A.9.2.1, information on chemicals of known toxicity which are included in the defined chemical class shall be reviewed. An appropriate number of chemicals of known toxicity shall be reviewed to establish class-based evaluation criteria. Sources of data for chemicals of known toxicity shall include, but not be limited to, the following:
– USEPA risk assessments, including Maximum Contaminant Levels (MCL), Health Advisories, and Integrated Risk Information System (IRIS) entries:
– Health Canada risk assessments;
– risk assessments previously performed to the requirements of annex A;
– state or provincial drinking water standards and guidelines; and
– World Health Organization (WHO) or other international drinking water standards and guidelines.
An MCL and SPAC (regulated contaminants) or a TAC and SPAC (non-regulated contaminants) shall be identified for each chemical of known toxicity which is being used to determine the class-based evaluation criteria. Carcinogenic potential shall be evaluated using a quantitative structure-activity relationship program (e.g., Oncologic® or equivalent) to verify the carcinogenic potential of the chemical of unknown toxicity is no greater than that of the chemicals being used to define the class-based evaluation criteria.
A.9.2.3 Determination of the class-based evaluation criteria
After review of the available toxicity information specified in annex A, section A.9.2.2, the class-based evaluation criteria shall not exceed the lowest MCL or TAC and SPAC identified for the chemicals of known toxicity in the defined chemical class. These evaluation criteria shall be used as surrogates for the TAC and SPAC for each chemical of unknown toxicity which meets the specifications of the defined chemical class (see
A15annex A, section A.9.2.1), until such time as sufficient toxicity data are available to determine chemical-specific evaluation criteria.
The class-based evaluation criteria shall not be applied to any substance for which available data and sound scientific judgement, such as structure-activity relationship considerations, indicate that adverse health effects may result at the established class-based evaluation criteria concentrations. If, after a chemical class is defined and its evaluation criteria established, a substance of greater toxicological significance is identified within the class, the class-based evaluation criteria shall be re-evaluated and revised to the acceptable concentrations of the new substance.
NOTE – It is recommended that documentation supporting class-based evaluation criteria be subject to the external peer review requirements of annex A, section A.10.15.
This section establishes the minimum criteria for the documentation of the data review performed on each drinking water additive chemical which requires a new or updated assessment. The assessment shall include, but not be limited to, evaluation of the elements detailed in this section.
A.10.1 Abstract
A summary shall be provided of the following:
– overview of the key toxicology studies;
– rationale for the selection of the critical effect and the corresponding NOAEL or other endpoint for calculation;
– major assumptions used in the assessment and areas of uncertainty; and
– presentation of the RID, TAC, SPAC and STEL values.
A.10.2 Physical and chemical properties
The assessment shall define the following parameters for the substance, as applicable:
– chemical formula, structure, CAS number, and molecular weight;
– physical state and appearance;
– melting point or boiling point;
– vapor pressure;
– solubility in water;
– organoleptic properties (taste and odor thresholds):
– dissociation constant (pKa); and
– partition coefficients (octanol/water, air/water).
A.10.3 Production and use
The assessment shall review the method(s) of production of the substance, whether it is a synthetic or a naturally occurring substance, and the principal uses of the chemical. This includes any use as a water treatment chemical or a food additive (direct or indirect) and its presence in such products as medicines, personal care products or cosmetics.
A16A.10.4 Analytical methods
For each identified analytical method for the substance, the following shall be summarized:
– analytical matrix;
– sample preparation, if applicable;
– method of analysis;
– type of detector or the wavelength for spectroscopic methods; and
– detection limit.
A.10.5 Sources of human and environmental exposure
The assessment shall describe the substance's natural occurrence, if any, and its presence in food or other media. Human exposure from drinking water, food, and air shall be described, including occupational exposures. The major source(s) and route(s) of human exposure shall be identified.
A.10.6 Comparative kinetics and metabolism
All references describing the absorption, distribution, metabolism, and excretion of the substance shall be reviewed. Both human data (when available) and animal data shall be included.
A.10.7 Effects on humans
A summary of each relevant reference documenting human exposure to the substance that is used in the hazard assessment shall be provided. These exposures can include both case reports of incidental human exposure to the substance, and epidemiological studies which explore the association between human exposure and specific toxic endpoints. Primary literature references shall be reviewed whenever possible.
Supporting data or other studies not utilized in the hazard assessment can be summarized in tabular form.
A.10.8 Effects on laboratory animals and in vitro test systems
A summary of each key study of the substance in experimental animals or in vitro test systems that is used in the hazard assessment shall be provided. The references used shall meet established toxicity study guidelines, as defined in annex A, section A.4.1, or any deficiencies shall be clearly identified. Studies shall include, but are not limited to the following: single exposure, short-term exposure (repeated dose study of < 28 days), long-term and chronic exposure (repeated dose study of $ 28 days), genotoxicity, reproduction and developmental toxicity, immunotoxicity, and neurotoxicity. Primary literature references shall be reviewed whenever possible.
Supporting data or other studies not utilized in the hazard assessment can be summarized in tabular form.
A.10.9 Effects evaluation
The effects evaluation is intended to provided an overall summary of the data reviewed for the substance and describe its mode/mechanism of action, if possible. This evaluation also serves to define the level of hazard represented by exposure to the substance at relevant human concentrations. This evaluation shall contain three major elements: hazard identification (assessment), dose-response assessment, and exposure characterization.
A.10.9.1 Hazard identification
The hazard identification (assessment) shall identify and discuss the following issues:
– the key data which define the basis of the concern to human health;
A17– the characterization of the substance as carcinogenic or non-carcinogenic, the basis for this characterization, and the critical effect(s);
– the extent to which this characterization is a function of study design (e.g., adequate number of doses used, effects noted only at highest dose, study performed at the maximum tolerated dose);
– the conclusions of the key study(ies) and whether they are supported or conflicted by other data;
– the significant data gaps for the substance and any relevant non-positive data;
– the available human data (case reports or epidemiological studies), and how they support or do not support the conclusions from the key study(ies);
– the mechanism by which the substance produces the adverse effect(s) noted in the key study, and whether this mechanism is relevant to humans; and
– the summary of the hazard assessment including confidence in the conclusions, alternate conclusions which may also be supported by the data, significant data gaps, and the major assumptions used in the assessment.
A.10.9.2 Dose-response assessment
The dose-response assessment shall identify and discuss the following issues:
– the data used to define the dose-response curve and in which species the data were generated;
– if animal data were used, whether the most sensitive species was evaluated;
– if human data were used, whether positive and negative data were reported;
– whether the critical data were from the same route of exposure as the expected human exposure (drinking water), and if not, discuss whether pharmacokinetic data are available to extrapolate between routes of exposure;
– for non-carcinogens, the methodology employed to calculate the RfD and the selection of the uncertainty factors which were used;
– for carcinogens, the dose-response model selected to calculate the LED10 and the rationale supporting its selection; and
– document the RfD calculation (see annex A, section A.7.3).
A.10.9.3 Exposure characterization
The exposure characterization shall identify and discuss the following issues:
A18– the most significant source(s) of environmental exposure to the substance, and the relative source contribution of each;
– the population(s) most at risk of exposure, and identify highly exposed or sensitive subpopulations; and
– any issues related to cumulative or multiple exposures to the substance.
A.10.10 Risk characterization
A.10.10.1 TAC derivation
The TAC derivation shall contain an explanation of all factors contributing to the TAC calculation, including adjustment for sources of the substance other than water. The TAC calculation shall be based on the oral RfD calculated during the dose response assessment in annex A, section A.10.9.2. The TAC calculation shall include adjustment for significant contributions of the substance from sources other than water, e.g., food and air. In the absence of data to determine the drinking water contribution of a substance, a default drinking water contribution of 20% shall be applied.
A.10.10.2 STEL derivation
When a short-term exposure level is calculated for a substance, the calculation shall be based on the NOAEL or LOAEL of the selected study (as defined in annex A, section A.5) with adjustment for body weight and daily water consumption of the protected individual, including any sensitive subpopulations. The default body weight and water consumption shall reflect that of a child, in the absence of data which demonstrate that adults are more sensitive to the substance than children. A rationale for the selection of uncertainty factors used in the calculation shall also be provided.
A.10.11 Risk management (SPAC derivation)
The TAC calculation shall form the basis of the SPAC calculation. The SPAC is equal to the TAC for qualitative risk assessments. For quantitative risk assessments, the SPAC shall be calculated as a percentage of the TAC value, based on the estimated total number of sources of the substance in the drinking water treatment and distribution system. In the absence of these data, the SPAC shall be calculated as 10% of the TAC value (default multiple source factor of 10 to account for other sources of the substance in drinking water).
A.10.12 Risk comparisons and conclusions
A review of other evaluations of the substance performed by other organizations (international, national, state or provincial agencies, or other entities) shall be provided. Consistencies and differences between evaluations shall be noted. Any uncertainties in these evaluations shall be discussed. A summary of the overall risk of the substance shall be made, including a discussion about compounds of comparable risk (e.g., similar structure, chemical class) when possible.
A.10.13 References
An alphabetized list of all reviewed citations (both cited and not cited in the assessment) shall be provided in an established format such as that described in The Chicago Manual of Style.
A.10.14 Appendices
Supporting documents, complex calculations, data summary tables, unique definitions, and other pertinent information shall be included in appendices to the document.
A.10.15 Peer review
Risk assessments performed to the requirements of this annex shall undergo external peer review (USEPA 1998) by an independent group of individuals representing toxicological expertise in the regulatory, academic, and industrial sectors, with the exception of the following:
A20– substances evaluated using the threshold of evaluation (see annex A, section A.7.1);
– substances evaluated to a TAC of 10 μg/L using the qualitative approach and concluded to be non-genotoxic
A19(see annex A, sections A.4.2 and A.7.2); and
– non-regulatory criteria which have already undergone peer-review, such as USEPA IRIS assessments.
References
Barnes, D. G., Daston, G. P., Evans, J. S., Jarabek, A. M., Kavlock, R. J., Kimmel, C. A., Park, C., and Spitzer, H. L. (1995). Benchmark Dose Workshop: Criteria for Use of a Benchmark Dose to Estimate a Reference Dose. Regulatory Toxicology and Pharmacology 21, 296-306.
Dourson, M.L. (1994). Methods for establishing oral reference doses (RfDs). In Risk Assessment of Essential Elements. W. Mertz, C.O. Abernathy, and S.S. Olin (editors), 51-61, ILSI Press Washington, D.C.
Dourson, M.L., Felter, S.P., and Robinson, D. (1996). Evolution of science-based uncertainty factors in noncancer risk assessment. Regulatory Toxicology and Pharmacology 24(2), 108-120.
International Programme on Chemical Safety (IPCS) (1994). Environmental Health Criteria No. 170: Assessing human health risks of chemicals: Derivation of guidance values for health-based exposure limits. World Health Organization, Geneva.
Klimisch, H.-J., M. Andreae, and U. Tillman (1997). A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data. Regulatory Toxicology and Pharmacology 25, 1-5.
Meek, M.E., Newhook, R., Liteplo, R.G., and V.C. Armstrong (1994). Approach to assessment of risk to human health for priority substances under the Canadian Environmental Protection Act. Environmental Carcinogenesis and Ecotoxicology Reviews C12(2), 105-134.
Renwick, A.G. (1993). Data derived safety factors for the evaluation of food additives and environmental contaminants. Food Additives and Contaminants. 10(3), 275-205.
TERA (Toxicology Excellence for Risk Assessment). 1996. Evolution of Science-Based Uncertainty Factors in Noncancer Risk Assessment. Full Report Prepared for Health and Environmental Sciences Institute (HESI), Cincinnati, Ohio, Jan. 31, 1996.
The Chicago Manual of Style. Chicago: The University of Chicago Press, 1982.
U.S. Environmental Protection Agency. 1993. Reference Dose (RfD): Description and Use in Health Risk Assessments. Background Document 1A. On-line Integrated Risk Information System (IRIS).
U.S. Environmental Protection Agency. 1995. Risk Assessment Forum. The Use of the Benchmark Dose Approach in Health Risk Assessment. EPA/630/R-94/007.
U.S. Environmental Protection Agency. 1994. Glossary of Risk Assessment Related Terms. On-Line Integrated Risk Information System (IRIS).
U.S. Environmental Protection Agency. 1999. Glossary of IRIS Terms. On-Line Integrated Risk Information System (IRIS).
U.S. Environmental Protection Agency. 1996a. Proposed Guidelines for Carcinogen Risk Assessment. Federal Register. 61(79): 17960-18011.
U.S. Environmental Protection Agency. 1991. National Primary Drinking Water Regulations; Final Rule. Federal Register. 56(20):3526-3614.
U.S. Environmental Protection Agency. 1998. Science Policy Handbook. Peer Review. EPA/100/B-98/001.
U.S. Environmental Protection Agency. 1999. Implementing the Food Quality Protection Act; Progress Report. EPA 735-R-99001.
U.S. Food Quality Protection Act. 1996. 7 USC 136.
U.S. Safe Drinking Water Act. 1996. Section 1412(b)(7)(A).
A21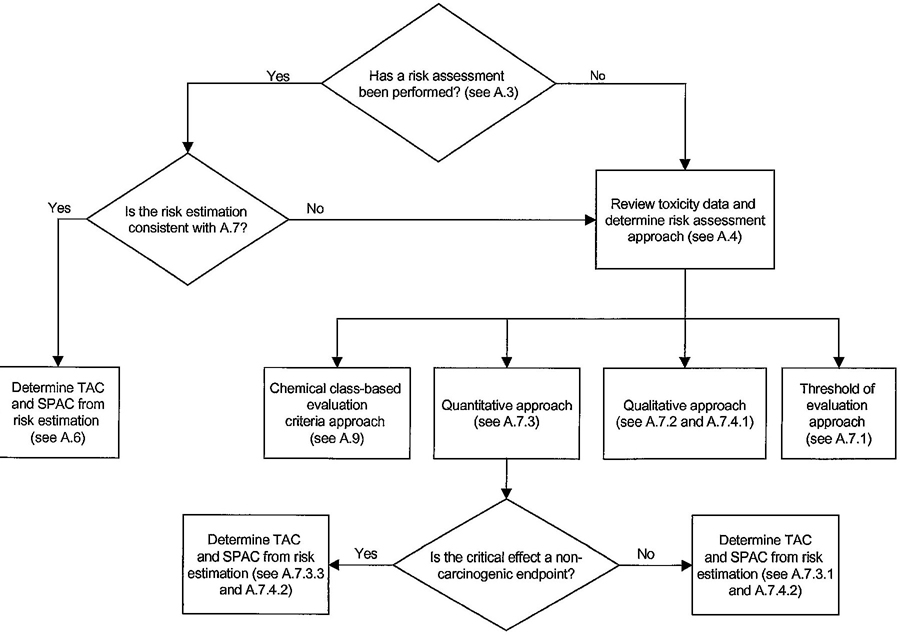
Figure A1-Annex A toxicity data review process
A22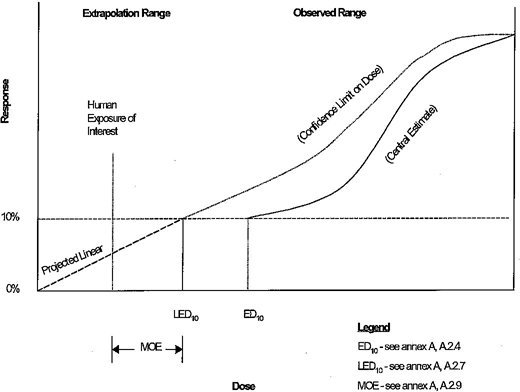
Figure A2-Graphical presentation of data and extrapolations (U.S. EPA, 1996a)
A23| Study type | Preferred criteria |
|---|---|
| Required studies | |
| Gene mutation assay1) | Bacterial reverse mutation assay performed with and without exogenous metabolic activation using Salmonella typhimurium (preferred strains are TA97, TA98, TA100, TA102, TA1535, and TA1537) or Escherichia coli (preferred strains are WP2 uvrA or WP2 uvrA (pKM101) |
| Chromosomal aberration assay1) (In vitro preferred) (In vivo) |
Metaphase analysis in mammalian cells and without exogenous metabolic activation Metaphase analysis or micronucleus assay in mammalian species |
| Supplemental studies | |
| Supplemental genotoxicity studies | Mouse lymphoma assay, SCE2), UDS3), HGPRT4), DNA binding (post labeling assay) |
| Bioaccumulation potential | Octanol/water partition coefficient |
| Pharmacokinetics | Absorption, distribution, metabolism, and excretion data in humans, other mammalian species, or both |
| Structural/functional assessment | Structure/activity relationship analysis |
| Acute or short-term toxicity5) | 1-to 14-day study or 14- to 28-day study using oral exposure route |
| Cell proliferation/cell cycle assays | Proliferating cell nuclear antigen (PCNA) |
| Sensitization | Guinea pig intradermal injection |
| In vivo gene mutation assay | Transgenic gene mutation assays |
| Endocrine disruption assays | Receptor binding/transcriptional activation assays, frog metamorphosis assay, steroidogenesis assay |
| Human data | Epidemiological, occupational, or clinical studies |
| 1) The gene mutation assay and the chromosomal aberration assay (in vitro or in vivo) shall constitute the minimum data set required to perform a qualitative risk assessment. When one or both in vitro genotoxicity studies are positive, the in vivo assay shall be required to be reviewed. 2) Sister chromatid exchange assay; SCEs are not considered to be mutagenic effects because the exchange is assumed to be reciprocal with no gain, loss, or change of genetic material. However, they do indicate that the test material has interacted with the DNA in a way that may lead to chromosome damage. In in vitro studies, SCEs do not provide adequate evidence of mutagenicity, but do identify the need for definitive chromosomal aberration studies. When evidence of in vitro clastogenicity exists, the induction of SCEs is often used as evidence of likely in vivo clastogenic activity because the in vitro aberration data demonstrate the clastogenic activity of the compound and the in vivo SCE data demonstrate that the compound interacted with the DNA in the target tissue. 3) Unscheduled DNA synthesis assay 4) Hypoxanthine guanine phosphoribosyl transferase assay 5) Minimum reported parameters shall include clinical observations, hematology and clinical chemistry, and gross pathology. |
|
| Study type | Preferred criteria |
|---|---|
| Required studies | |
| Gene mutation assay1) | Bacterial reverse mutation assay performed with and without exogenous metabolic activation using Salmonella typhimurium (preferred strains are TA97, TA98, TA100, TA102, TA1535, and TA1537) or Escherichia coli (preferred strains are WP2 uvrA or WP2 uvrA (pKM101) |
| Chromosomal aberration assay1) (In vitro preferred) | Metaphase analysis in mammalian cells and without exogenous metabolic activation |
| (In vivo) | Metaphase analysis or micronucleus assay in mammalian species |
| Subchronic toxicity1) | 90 day assay in rodent species by oral route of exposure |
| Additional studies (required as indicated) | |
| Reproduction assay2) | Two generation reproductive assay in a rodent species |
| Developmental assay2) | Teratology study (two species, one rodent and one non-rodent, are preferred) |
| Chronic study3) | Two-year bioassay in rodent species by oral route of exposure |
| Supplemental studies | |
| Supplemental genotoxicity studies | Mouse lymphoma, SCE4), UDS5), HGPRT6), DNA binding (post labeling assay) |
| Bioaccumulation potential | Octanol/water partition coefficient |
| Pharmacokinetics | Absorption, distribution, metabolism, and excretion data in humans, other mammalian species, or both |
| Structural/functional assessment | Structure/activity relationship analysis |
| Acute or short-term toxicity7) | 1-to 14-day or 14- to 28-day study using oral exposure |
| Cell proliferation/cell cycle assays | Proliferating cell nuclear antigen (PCNA) |
| Sensitization | Guinea pig intradermal injection |
| In vivo gene mutation assay | Transgenic gene mutation assays |
| Endocrine disruption assays | Receptor binding/transcriptional activation assays, frog metamorphosis assay, steroidogenesis assay |
| Human data | Epidemiological, occupational, or clinical studies A25 |
| 1) The gene mutation assay, the chromosomal aberration assay (in vitroor in vivo), and the subchronic toxicity study shall constitute the minimum data set required to perform a quantitative risk assessment. When one or both in vitro genotoxicity studies are positive, the in vivo assay shall be required to be reviewed. 2) It is recommended that results of a screening assay, such as OECD No. 422, Combined repeated dose toxicity study with reproductionl developmental toxicity screening test, or data from other repeated dose assays which include histopathological examination of the reproductive tissues of each sex be reviewed prior to a determination that these assays are required for evaluation. 3) A chronic study with evaluation of carcinogenic endpoints is required when review of the minimum data set concludes that the substance is likely to be a human health hazard at exposures of 10 μg/L or less. 4) Sister chromatid exchange assay; SCEs are not considered to be mutagenic effects because the exchange is assumed to be reciprocal with no gain, loss, or change of genetic material. However, they do indicate that the test material has interacted with the DNA in a way that may lead to chromosome damage. In in vitro studies, SCEs do not provide adequate evidence of mutagenicity, but do identify the need for definitive chromosomal aberration studies. When evidence of in vitro clastogenicity exists, the induction of SCEs is often used as evidence of likely in vivo clastogenic activity because the in vitro aberration data demonstrate the clastogenic activity of the compound and the in vivo SCE data demonstrate that the compound interacted with the DNA in the target tissue. 5) Unscheduled DNA synthesis assay 6) Hypoxanthine guanine phosphoribosyl transferase assay 7) Minimum reported parameters include clinical observations, hematology and clinical chemistry, and gross pathology. |
|
| Conclusion of data review | TAC |
|---|---|
| The weight of evidence review of the required genotoxicity studies and all other relevant data concludes that the substance is not a hazard at exposures of 10 μg/L or less. | 10 μg/L |
| The weight of evidence review of the required genotoxicity studies, a repeated dose study of less than 90 days duration1), and all other relevant data concludes that the substance is not a human health hazard at exposures of 50 μg/L or less. | # 50 μg/L |
| The weight of evidence review of the required genotoxicity studies and all other relevant data concludes that the data are insufficient to determine the potential human health hazard of the substance at exposures of 10 μg/L or less. | Supplemental studies or chronic toxicity and carcinogenesis bioassay required for review |
| The weight of evidence review of the required genotoxicity studies and all other relevant data concludes that the substance is likely to be a human health hazard at exposures of 10 μg/L or less. | Chronic toxicity and carcinogenesis bioassay required for review |
| 1) Required study parameters include organ and body weights, clinical chemistry and hematology, gross pathology, and histopathology. | |
| Areas of uncertainty | Factor |
|---|---|
| Intraspecies extrapolation (species variation): This factor accounts for variations in chemical sensitivity among individuals in a species including toxicokinetic and toxicodynamic parameters. | 1, 3, or 10 |
| Interspecies extrapolation (animal to human): This factor accounts for variations in chemical sensitivity between experimental animals and humans, including toxicokinetic and toxicodynamic parameters. | 1, 3, or 10 |
| Less than lifetime duration of exposure: This factor is intended to extrapolate experimental results from subchronic to chronic exposure. | 1, 3, or 10 |
| Use of LOAEL rather than NOAEL1): This factor addresses the uncertainty in developing a reference dose from a LOAEL rather than a NOAEL. | 1, 3, or 10 |
| Lack of database completeness: This factor accounts for the absence of data for specific toxic endpoints. | 1, 3, or 10 |
1) This adjustment is not required for BMD calculations.
|
|
Products/materials to be evaluated shall be prepared, exposed, and the extraction medium (e.g., water, chemical) analyzed as described in this annex. Examples of products/materials covered by this annex are shown in annex B, table B1.
Table B2 outlines the various preparation and exposure methods for the products/materials covered by the annex.
The analytical methods included are based on contaminants that are likely to be present when established methods of production are used and the materials are derived from known sources. Modifications to the analytical procedures shall be permitted when products/materials are produced with alternate methods or have originated from alternate sources.
B.2.1 General
The requirements described in this section are general requirements and apply to all products/materials covered by Standard 61. Annex B, sections B.3 to B.5 describe specific preparation, conditioning, and exposure sequences unique to individual product/material categories.
B.2.2 QA/QC and safety
The methods included in annex B, sections B.3 to B.5 have been written for trained chemical laboratory personnel. Appropriate quality assurance procedures and safety precautions shall be followed.
B.2.3 Samples
B.2.3.1 Material evaluation
A representative sample of the material (in either material sample or finished product form) shall be exposed.
B.2.3.2 Finished product evaluation
Samples of the finished product (e.g. pipe, fitting, device) shall be exposed except in the following specific instances:
– concrete cylinders, cubes, or other concrete surrogate samples shall be permitted to be evaluated on behalf of concrete lined pipes and other concrete-based products;
– coatings, applied to an appropriate substrate, shall be permitted to be evaluated on behalf of products whose entire water contact surface is covered by the coating; and
– finished products, for which finished product evaluation is impractical due to one or more of the following reasons, shall be permitted to be evaluated using material samples:
– an internal volume greater than 20 L (5.3 gal);
– a weight greater than 34 kilos (75 lbs.);
B1– in situ manufacture of the finished product.
Material samples shall be permitted to be evaluated on behalf of a finished product if, and only if, no chemical or physical difference exists between the material sample and the material as represented in the finished product. All material samples shall be produced using all of the same manufacturing processes as the finished product.
B.2.4 Washing
To remove any extraneous debris or contamination that occurred during shipping and handling, samples shall be rinsed with cold tap water prior to testing, followed by a deionized water (ASTM D1193 Type II) rinse, unless the manufacturer’s instructions direct otherwise. If the exterior of a product is exposed, any printed markings (e.g., ink markings) shall be removed.
B.2.5 Extraction waters
Samples shall be exposed, based on a formulation review and determination of the most severe condition(s), to one or more extraction waters as detailed in table B3, except for mechanical plumbing devices (annex B, section B.5.5). The characteristics and preparation of the waters are described in annex B, section B.9.
B.2.5.1 Exceptions
The manufacturer shall have the option to specifically request a change in the extraction water used, based on the intended application or the materials used in the device/product, provided the manufacturer’s use instructions indicate the use limitations.
B.2.5.2 Mechanical devices used in contact with drinking water treatment chemicals
These devices and materials shall be exposed to the chemicals and chemicals mixtures that have been specified by the manufacturer.
B.2.5.3 Copper and copper alloys
Pipe and tubing manufactured from copper alloy C12200 shall be exposed in the pH 6.5 (annex B, section B.9.4) and in the pH 10 (annex B, section B.9.7) extraction waters. The manufacturer’s use instructions shall indicate this use limitation.
Copper and copper alloy fittings intended to be used used with copper pipe and tubing shall be exposed in either the pH 5 or the pH 6.5 exposure waters (at the discretion of the manufacturer) and in the pH 10 exposure water. When electing to use of pH 6.5 exposure water, the manufacturer’s literature shall indicate this use limitation.
B.2.6 Product exposure
Samples shall be evaluated either “in-the-product/device” or in an exposure vessel.
B.2.6.1 Exposure in-the-product/device
When practical, products/devices shall be evaluated such that only the (exposed) wetted surface is exposed to extraction medium.
B.2.6.2 Exposure in vessels
Samples that are not evaluated as described in annex B, section B.2.6.1 shall be exposed to the extraction medium in containers composed of a material that is inert to the exposure water and with PTFE (polytetrafluoroethylene) lined lids, with no headspace.
B2Products exposed in vessels shall be exposed such that the surface area-to-volume ratio described in the appropriate section (annex B, section B.3 to B.5) shall be maintained.
B.2.6.3 Residual vinyl chloride monomer (RVCM)
Polyvinyl chloride (PVC) and chlorinated polyvinyl chloride (CPVC) pipe products/materials shall be evaluated for RVCM. RVCM shall be determined in the product wall, rather than by extraction, in accordance with annex B, section B.7.
B.2.7 Material exposure
Materials shall be exposed according to the protocol outlined for the materials’ specified end use(s). If a material is intended for use in the manufacture of products covered under more than one section of this standard, the most stringent exposure condition shall be followed (e.g temperature, surface area-to-volume ratio). A material intended to be processed to be covered by more than one method (e.g. injection molding, extrusion, stamping) shall be tested in each of the processed forms.
B.2.7.1 Exposure of a material sample
A materials manufacturer shall have the option to request that a material be tested as a material sample (e.g., plaque, sheet) if, and only if, there is no chemical or physical difference in the material characteristics between the material sample and the material as it is used in covered applications. If the material is intended to be used only for the manufacture of products falling under the scope of a single section of this standard, the material shall be exposed under the conditions set forth in the corresponding section of annex B. The normalized contaminant concentrations shall meet the requirements of an annex A.
B.2.7.2 Exposure in product form
A materials manufacturer shall have the option to request that a material be tested in the form of a finished product according to the protocol set forth in the appropriate section(s) of annex B.
B.2.7.3 Surface area-to-volume ratio (s/v)
When testing the material in the form of a material sample or in product form, the dimensions of the material or the product sample tested and the extraction medium volume shall be recorded and the laboratory tested surface area-to-volume ratio calculated. When necessary, laboratory extraction results shall be adjusted to reflect the difference between laboratory and field surface to area volume ratios.
B.2.8 Exposure conditions
Exposure begins immediately following washing or the appropriate conditioning.
B.2.8.1 Method blanks
Method blanks shall be prepared using the same reagent and in the same manner as product samples, but no product shall be added. An uncoated substrate, as applicable, shall be included. Methods blanks shall be processed with all samples.
B.2.8.2 Method standards.
Method standards shall be prepared along with all samples. Method standards are prepared in the same manner as method blanks, except a known amount of the expected contaminant is added.
B3B.2.8.3 Sequential exposure
Tests for evaluation shall be conducted using a sequential exposure procedure. There shall be no significant time interval between exposures (decant, discard, fill, continue exposure). The products shall be exposed depending on the intended and use application, as described in the appropriate section (annex B, sections B.3 to B.5). Analyses shall be performed only on the final extraction medium, unless otherwise noted.
B.3.1 Sample requirements
Test samples of joining and sealing materials shall be prepared such that, upon exposure, a minimum surface area-to-volume ratio of 15 cm2/L (8.8 in2/gal) is obtained. Materials used at higher surface to volume ratios in the field shall be exposed at the actual use ratio or greater. Test samples for the various types of joining and sealing materials are described in annex B, table B4.
B.3.2 Preparation
Samples shall be prepared so that the entire surface to be exposed is covered by extraction water. Products (as appropriate) shall be applied to a glass panel in a manner consistent with the manufacturer’s published instructions. Products requiring a reactive substrate (i.e., when glass is inappropriate), shall be applied to an appropriate alternate substrate.
B.3.2.1 Gasket materials
These products shall be cut to the appropriate size as described in annex B, section B.3.1.
B.3.2.2 Caulks, greases, lubricants, and sealants
These products shall be applied to a glass panel in such a manner that an even film, consistent with end use, is exposed and the surface area-to-volume ratio described in annex B, section B.3.1 is maintained. The slides shall be allowed to air dry or cure according to the manufacturer’s published instructions.
B.3.2.3 Adhesives and cements
B.3.2.3.1 Adhesives and cements intended for joining pipe and fittings shall be prepared as pipe and fittings joints assembled in accordance with the manufacturer’s use instructions. The joints shall be produced using ½ nominal diameter pipe (or tubing) and fittings, or the minimum size specified by the manufacturer, if it is greater. Unless otherwise stated in the manufacturer’s use instructions, PVC pipe and fitting joints shall be assembled per ASTM D2855 and CPVC pipe and fitting joints assembled per appendix XI of ASTM F493. If the manufacturer’s use instructions recommend use of a primer, testing shall incorporate use of a primer. Unless otherwise stated in the manufacturer’s use instructions, joints shall be allowed to air cure for 48 hours (± 2 hours) at room temperature prior to washing, conditioning, and exposure in-product.
B.3.2.3.2 Adhesives and cements not intended for joining pipes and fittings shall be prepared in a manner consistent with the manufacturer’s use instructions. These products shall be applied to glass panels (or manufacturer’s intended substrate) such that an even film, consistent with end use, is exposed at a field surface area-to-volume ratio greater than or equal to a typical installation. Unless otherwise indicated in the manufacturer’s use instructions, the slides shall be allowed to air cure for 48 hours (± 2 hours) at room temperature prior to washing, conditioning, and exposure in-vessel.
B4B.3.2.4 Solders
These products shall be prepared by placing the solder in a ceramic combustion boat (96 mm × 12 mm × 10 mm). The amount of solder used shall be sufficient to cover the bottom of the boat. The boat (with solder) is then placed in a muffle furnace that has been set to a temperature which is 20°C (36°F) above the liquids temperature of the product being evaluated. For example:
95/5 tin/antimony soldier has a melting range of 232°C–240°C (450°F–464°F). The oven is set at 260°C (500°F) for this solder.
The boat (with solder) is placed in the oven and allowed to heat until the solder has melted (approximately 1-2 minutes). The boat is allowed to cool and the solder piece is removed.
B.3.2.5 Fluxes
These products shall be prepared by applying a thin film to a copper sheet of the appropriate size as described in annex B, section B.3.1. The copper sheet is then placed in a muffle furnace that has been set to 300°C (572°F). The copper sheet (with flux) is allowed to heat until the flux flows (approximately 30 seconds to 1 minute). The copper sheet is allowed to cool prior to exposure.
B.3.3 Conditioning for joining and sealing materials intended for joining pipe and fittings
Following washing (annex B, section B.2.4), and prior to exposure, product/material samples shall be conditioned to simulate pre–use flushing and disinfection, procedures. The samples shall be exposed for evaluation immediately after conditioning. Joining and sealing materials shall be conditioned at the temperature appropriate for the intended end use. The product samples shall be conditioned as described in annex B, sections B.3.3.1 or B.3.3.2.
B.3.3.1 Cold application
Following washing (annex B, section B.2.4), the samples shall be conditioned at room temperature (23±5°C [73±9°F) with the applicable pH 8 extraction water for 14 days, or less if specified by the manufacturer. The water shall be changed at least ten times, with a minimum period of 24 hours per conditioning period. There shall be no time interval between exposures. To simulate disinfection, the first (initial) exposure shall contain 50 mg/L available chlorine. After the initial 24-hour period, the extraction water shall contain 2 mg/L chlorine.
B.3.3.2 Hot application
Following washing (annex B, section B.2.4), and the cold application conditioning as described in annex B, section B.3.3.1, the samples shall be further conditioned at the elevated temperature by exposure with the applicable pH 8 extraction water using the sequence in annex B, table B5. There shall be no time interval between exposures.
B.3.4 Conditioning for all other joining and sealing materials
Following preparation, the test samples are washed as described in annex B, section B.2.4.
B.3.5 Exposure for joining and sealing materials intended for joining pipe and fittings
Exposure shall begin immediately following conditioning. The samples shall be exposed to the appropriate extraction water, annex B, section B.2.5, based on end use or application. The extraction water shall be collected for analysis as described in annex B, section B.6.
B5B.3.5.1 Cold application
Products which, in actual field use, are not used with hot water shall be exposed as follows: 16 hours ±1 hour at 30± 1°C (86 ± 4°F).
B.3.5.2 Hot application
Products which, in actual field use, are used with hot water shall be exposed as follows:
Exposed for 0.5 hour ± 5 minutes at 82 ± 0.5°C (180 ± 1°F), immediately followed by exposure for 16 ± 1 hour at 30± 1°C (86 ± 4°F).
The test sample shall be allowed to cool in the 30°C (86°F) chamber. Successful evaluation using this procedure shall preclude the cold exposure.
B.3.6 Exposure for all other joining and sealing materials
Following conditioning, these materials shall be exposed in the appropriate extraction water, annex B, section B.2.5, in accordance with the intended end use application as described below. The extraction water samples shall be collected as described in annex B, section B.6.
B.3.6.1 Cold application
Products to be evaluated for cold applications shall be exposed using the following sequence:
B.3.6.2 Hot application samples
Products to be evaluated for hot applications shall be exposed using the sequence that follows. However, the manufacturer shall be permitted to specify an alternate maximum temperature.
B.3.7 Extended exposure
If the normalized concentration of a contaminant exceeds the annex A criteria (chronic exposure), the manufacturer shall be permitted to request that additional exposure testing be undertaken. The additional testing shall be used to determine a contaminant leaching rate(s) over time. The relationship between contaminant concentration and time shall be determined, plotted with a minimum of five points, and then used for evaluation. When feasible, testing shall continue until contaminant concentrations are reduced to ten percent of the initial contaminant concentration. The normalized concentrations shall then be compared to the SPAC as specified in annex A, section in annex A, section A.5 (potential exposure resulting from short-term high level leachates).
B6B.4.1 Samples
Samples shall consist of the entire device, or portion(s)/component(s) of the device, or a specimen of the material(s). The manufacturer shall have the option to request that the samples represent a product line of varying sizes, as described below. When it is necessary to calculate normalization factor(s), the wetted exposed surface area of the sample shall be calculated and recorded prior to testing.
B.4.1.1 Entire device
A single device shall represent a product line of varying sizes when:
– materials are of the same alloy, composition, or formulation; and
– designs are analogous; and
– it has the greatest exposed wetted surface area-to-volume ratio.
The wetted surface area-to-volume ratio shall be calculated as SAF/VF(static), with SAF equal to the surface area exposed in the field, and VF(static) equal to the volume of water to which the device is exposed under the static condition. The surface area-to-volume ratio for a device having an internal volume of less than 1 L (0.26 gal) shall be calculated with the assumption that VF(static) is equal to 1 L.
NOTE – For a product line of varying sizes having volumes of less than 1 L, the device with the largest wetted surface area will be the device having the greatest exposed surface area-to-volume ratio.
B.4.1.2 Component
A component shall represent a product line of varying sizes when:
– materials are of the same alloy, composition, or formulation; and
– designs are analogous; and
– it has the greatest exposed wetted surface area-to-volume ratio.
The wetted surface area-to-volume ratio shall be calculated as SAF/VF(static), with SAF equal to the surface area exposed in the field, and VF(static) equal to the volume of water to which the component is exposed under the static condition. The surface area-to-volume ratio for a component having an internal volume of less than 1 L (0.26 gal) shall be calculated with the assumption that VF(static) is equal to 1 L.
NOTE – For a product line of varying sizes having volumes of less than 1 L, the component with the largest wetted surface area will be the component having the greatest exposed surface area-to-volume ratio.
B.4.1.3 Material
The material shall be representative of that used in the component or device.
Materials shall be evaluated using a minimum surface area-to-volume ratio of 50 cm2/L.
B7B.4.2 Washing
Prior to conditioning and exposure, the samples shall be washed as described in annex B, section B.2.4, unless the manufacturer’s instructions direct otherwise. When required, the device shall be properly prepared per the manufacturer’s recommendations.
B.4.3 Conditioning
Conditioning shall be conducted either in the device or in a vessel. Table B6 provides examples of typical exposures for the various products covered by this section. The test samples shall be preconditioned by exposure at room temperature 23 ± 2°C (73 ± 4°F) to the extraction water used for testing (annex B, section B.2.5) for 14 days or less if specified by the manufacturer. The water shall be changed at least 10 times (during the 14-day conditioning period), or less if specified by the manufacturer. There shall be a minimum period of 24 hours per exposure.
B.4.4 Exposure
Following conditioning, the samples shall be exposed as described in annex B, table B7 in the appropriate extraction media (annex B, section B.2.5). Devices/components which in actual field used with hot water (e.g., distribution system valves), shall be exposed using the sequence shown in table B7. Devices/components which are used in contact with water at a temperature in excess of 23°C (73°F) shall be exposed using the same exposure sequence, at the maximum temperature encountered under use conditions. The extraction media shall be collected as described in annex B, section B.6.
B.4.5 Chemical exposure
The samples shall be exposed to the appropriate drinking water treatment chemical or chemical mixture for a minimum of 4 hours (or for a longer period as recommended by the manufacturer) at 23± 2°C (73 ± 4°F). For devices that normally operate at lower or higher temperatures, the exposure shall be at normal operating temperature. The extractant shall be collected in a vessel appropriate for shipping and storage. For chemical feeders, a sample of the chemical prior to feeding shall be collected if possible. For chemical generators, samples of the raw chemicals shall be collected. For all devices where the extractant is a mixture of water and the chemical(s), a sample of the influent water shall be collected and preserved as described in annex B, section B.6. Analysis of the extractant shall be in accordance with the requirements of NSF/ANSI 60. Samples of the chemicals prior to feeding samples of raw materials, and influent water samples shall be analyzed for background levels of contaminants only if, after normalization, the concentration of a contaminant(s) exceeds the SPAC (annex B, section B.8.6).
B.5.1 Samples
Samples shall consist of the entire device, portion(s)/component(s) of the device, or a specimen of the materials(s) of the device. The samples shall be permitted to represent a product line of varying sizes, as described in B.5.1.1 and B.5.1.2. When it is necessary to calculate normalization factor(s), the wetted surface area of the sample shall be determined. When testing materials and components using in–vessel exposure, the actual wetted surface area and the volume of water in the extraction vessel shall be determined.
B.5.1.1 Device
A single device shall represent a product line of varying sizes when the following requirements are met:
– materials are of the same alloy, composition, or formulation;
– design and manufacturing processes are analogous; and
B8– it has the greatest wetted surface area-to-volume ratio.
The surface area-to-volume ratio for an endpoint device, other than a commercial kitchen device, shall be calculated with the assumption that the device volume is 1 L (0.26 gal). The surface area-to-volume ratio for a commercial kitchen device shall be calculated with the assumption that the device volume is 18.9 L (5 gal).
B.5.1.2 Component
A component shall represent a product line of varying sizes when the following requirements are met:
– materials are of the same alloy, composition, or formulation;
– design and manufacturing processes are analogous; and
– it has the greatest wetted surface area-to-volume ratio.
The surface area-to-volume ratio for a regular endpoint component shall be calculated with the assumption that the component volume is 1 L (0.26 gal).
B.5.1.3 Material
The material shall be representative of that used in the component or device. Material samples not related to a specific component or device can also be evaluated.
B.5.2 Washing
Flush device for 15 minutes with tap water under pressure, then rinse with 3 volumes of DI water (ASTM D–1193, Type II). Alternate preparation of the device shall be performed when required by published manufacturer’s instructions. Components and materials shall be washed according to annex B, section B.2.4.
B.5.3 Conditioning
Conditioning of the sample shall be performed in the sample or in a vessel. Endpoint devices, components, and materials shall be conditioned by rinsing with 3 volumes of extraction water (specified in annex B, section B.5.5) at room temperature 23 ± 2EC (73 ± 4EF). The units of exposure vessels shall be filled with extractant water and held until the start of the exposure sequence for a period not to exceed 72 hours.
B.5.4 Exposure
Following conditioning, the sample shall be exposed to extraction water according to the applicable scheme detailed in annex B, sections B.5.4.1 through B.5.4.3. Reflecting the sample’s intended use, samples shall be exposed to extraction waters at the specified temperatures for the entire duration of the exposure. Exposure shall be limited to 23 ± 2EC (73 ± 4EF) except for instant hot water dispensers, in which case the manufacturer’s specified thermostat setting shall be used.
Evaluation of endpoint devices, components, and materials for contaminates other than lead shall require exposure of at least one sample according to the timetable of figure B1. The number of products to be tested shall be specified by the manufacturer. When one sample is tested, the normalized contaminant concentrations from exposure on Day 19 shall be compared to their respective SPACs. If more than one sample is tested, the geometric mean of normalized contaminant concentrations from exposure on Day 19 shall be compared to their respective SPACs.
Evaluation of endpoint devices, components and materials for the contaminant lead shall require exposure of at least 3 devices (more if specified by the manufacturer), according to the timetable of figure B1. It is recommended that product lines thought to be marginally acceptable, and those that leach levels of lead approaching the maximum allowable level, should be tested for more than the minimum number of products. The rationale for selecting a number greater than 3 products is provided in annex B, section B.8.9. On Days
B93, 4, 5, 10, 11, 12, 17, 18, and 19, the 16-hour dwell extractant water shall be collected. The lead test statistic Q shall be determined as described in annex B, section B.8.9 and compared to 11 μg.
When additional extraction water is needed to complete all analyses, additional samples shall be exposed.
B.5.4.1 Exposure sequence for endpoint devices
The device shall be inverted and filled with extraction water and held according to figure B1 during the exposure sequence. Hot water dispensers shall be heated to operating temperature, then exposed following the sequence in figure B1 at the elevated temperature.
The final exposure water shall be collected and preserved in accordance with applicable analytical methods. Only the contaminant levels present in the 16-hour dwell samples shall be used to evaluate the product’s leaching characteristics.
For endpoint devices, the exposure sequence in figure B1 shall be conducted and the Days 3, 4, 5, 10, 11, 12, 17, 18 and 19 lead dosages shall be determined.
B.5.4.2 Exposure sequence for components and materials
The exposure procedures provided in annex B, section B.5.4.1 shall be followed. Samples shall be tested at a surface area-to-volume ratio at least as high as the ratio that exists in the device.
B.5.4.3 Method blanks
Method blanks are prepared using the same reagents and in the same manner as samples, but no sample is added. An uncoated substrate, as applicable, shall be included. Method blanks shall be processed with all samples.
B.5.4.4 Method standards
Method standards shall be prepared along with all samples. Method standards are prepared in the same manner as method blanks, except a known amount of the expected contaminants is added.
B.5.5 Extraction water
The extraction water shall be prepared by combining:
– 25 ml of 0.4M sodium bicarbonate;
– chlorine stock solution as per annex B, section B.9.2.4;
– deionized water meeting specification ASTM D1193 Type II (make up to one liter), and adjust DH as needed using 0.1M HCI (approximately 1.3 ml) to meet the following requirements:
This water shall have a pH of 8.0 (± 0.5), alkalinity of 500 ppm (± 25 ppm), dissolved inorganic carbon of 122 ppm (± 5 ppm), and 2 ppm (± 0.5 ppm) of free chlorine.
Immediately following the exposure period, the extraction media shall be poured into sample bottles previously prepared as detailed in annex B, table B8 for storage until analysis. The procedures described in annex B, table B8 are based on collection methods included in “Manual For The Certification of Laboratories Analyzing
B10Drinking Water,” (EPA-570/9-82-002) and Standard Methods For The Examination of Water and Wastewater (most recent edition).
B.7.1 General
This section is divided into five parts: metals, organics, (other than residual vinyl chloride monomer (RVCM) and solvent), radionuclides, RVCM, and solvents analyses. The specific analyses performed shall be formulation dependent.
Each testing organization shall periodically review the analytical methods it uses to ensure that applicable advances in analytical methodologies are instituted.
B.7.2 Definitions
B.7.2.1 identified compound with standard: A compound identification made based on the daily analysis (initial or continuing calibration) of an authentic standard of an analyte. Retention time and mass spectrum are used for qualitative determination of the analyte. A calibration curve is used for quantitative determination of the analyte.
B.7.2.2 identified compound without standard: A compound identification based on mass spectral matches between the analyte and mass spectral libraries (commercial or private), or based on spectral interpretation by a qualified chemist, or both. The quantitative determination is made through direct correlation between the analyte response and the nearest internal standard response.
B.7.2.3 matrix spike: An aliquot of a sample matrix fortified with a known quantity of analyte.
B.7.2.4 method detection limit (MDL): As defined in 40 CFR Part 136, Appendix B, the minimum concentration of a substance that can be measured and reported with 99% confidence that the substance concentration is greater than zero. The MDL is determined from analysis of a minimum of seven aliquots of standard (known quantity of analyte in reagent matrix) at concentrations which are in the range of the estimated detection limit.
B.7.2.5 method validation: Verification of an analytical procedure performed by determining the method detection limit (see annex B, section B.7.2.4).
B.7.2.6 reporting limit (RL): The lowest concentration of analyte that can be reliably reported.
B.7.2.7 unknown: An analyte for which an identification cannot be determined. Information on chemical class, functional group(s), and chemical structure may be determined by spectral interpretation.
B.7.3 Metals analysis
Analyses for metals shall be performed, except as otherwise provided for herein, in accordance with currently accepted U.S. Environmental Protection Agency (EPA) Methods (see 40 CFR Part 141 and Methods for Chemical Analysis of Water and Wastes, EPA 600/4-79-020). When no EPA method is provided, analyses shall be performed in accordance with Standard Methods for the Examination of Water and Wastewater (most current edition). If neither of these two references address the required parameters and matrix, or if an alternate method is desired, method validation shall be completed prior to the application of the method (see annex B, section B.7.2.5).
B11B.7.4 Organics analysis
B.7.4.1 General requirements for analysis of organics
Analyses for organics shall be performed, except as otherwise provided for herein, in accordance with currently accepted EPA methods (see 40 CFR Part 141 and Methods for Chemical Analysis of Water and Wastes, EPA 600/4-79-020). When no EPA method is provided, analyses shall be performed in accordance with Standard Methods for the Examination of Water and Wastewater (most current edition). If neither of these two references address the required parameters and matrix, or if an alternate method is desired, method validation shall be completed prior to the application of the method (see annex B, section B.7.2.5).
B.7.4.2 Gas chromatography/mass spectroscopy (GC/MS) analysis
B.7.4.2.1 General requirements for GC/MS analysis
The minimum instrument operation requirements for GC/MS analysis shall be in accordance with USEPA Method 625 with the addition of the following modifications:
– The average chromatographic peak area of each internal standard in the calibration curve shall be determined. The chromatographic peak area of each internal standard in the continuing calibration shall be greater than 50% and not more than 200% of that average;
– While performing a continuing calibration check (CCC), concentrations of 10% of the target compounds for each analysis (e.g. base/neutral, base/neutral/acid, acid) shall be allowed to fall outside of the range of 70% to 130% (outlier) of the true value. None of the concentrations shall be allowed to fall below 50% or above 200% of the true value. If a positive sample analyte result is identified for any outlier, a second CCC shall be performed. If the second CCC determines the sample analyte result to no longer be an outlier, the sample shall be reanalyzed. However, if the second CCC also determines the analyte to be an outlier, a new calibration curve shall be determined and the sample shall be reanalyzed;
– If commercially available mass spectral libraries are utilized, a minimum size of 100,000 compounds shall be required; and
– The testing laboratory shall report the compounds detected during GC/MS analysis as one of the following:
– identified compound with standard (see annex B, section B.7.2.1);
– identified compound without standard (see annex B, section B.7.2.2); or
– unknown (see annex B, section B.7.2.7).
B.7.4.2.2 Requirements for identified compounds with standards via GC/MS analysis
Contaminants which have been identified and quantified by comparison to authentic standards shall be normalized in accordance with the requirements of this Standard (see annex B, section B.8). The normalized contaminant concentration shall be compared to the acceptable exposure concentration as determined in accordance with annex A of this Standard.
B.7.4.2.3 Requirements for Identified compounds without standards via GC/MS analysis
Contaminants which have been identified and quantified by comparison to a known mass spectrum, or by spectral interpretation by a qualified chemist, or both, shall be normalized in accordance with the requirements of this Standard (see annex B, section B.8). The normalized contaminant concentration shall be compared to
B12the acceptable exposure concentration as determined in accordance with annex A of this Standard. In addition, the product manufacturer shall assist the testing laboratory in the identification of an authentic standard for the compound and an appropriate analytical method, if applicable, so that confirmatory identification and quantification can be performed.
B.7.4.2.4 Requirements for unknowns via GC/MS analysis
Contaminants detected by GC/MS analysis, but which are not identified and quantified against a known mass spectrum or authentic standard, shall be evaluated as follows:
a) The product material formulation(s) shall be reviewed for potential identification of the unknown contaminant(s) as an ingredient or by-product;
b) The manufacturer shall be notified and requested to provide supporting information that enables identification of the unknown contaminant(s);
c) Structure activity relationships (SAR) shall be utilized when sufficient structural identification of the unknown contaminant(s) can be made; and
d) Alternative methods of analysis that may identify the unknown contaminant(s) shall be considered.
Contaminants which can be identified after performing one or more of the above steps shall be normalized in accordance with the requirements of this Standard (see annex B, section B.8). The normalized contaminant concentration shall be compared to the acceptable exposure concentration as determined in accordance with annex A of this standard. In addition, the product manufacturer shall assist the testing laboratory in the identification of an authentic standard for the compound and an appropriate analytical method, if applicable, so that confirmatory identification and quantification can be performed.
Contaminants detected by GC/MS analysis for which no identification can be made after performing the above steps shall not be considered in the determination of product compliance to this standard. When unknown contaminants are detected in the extractant water, the testing laboratory shall report the analytical results to the product manufacturer.
NOTE – The product manufacturer should assist the testing laboratory in a continuing effort to identify the unknown contaminant(s) until specific identification is achieved.
B.7.4.3 Polynuclear aromatic hydrocarbon (PNA) analysis
Analysis for polynuclear aromatic hydrocarbons (PNAs) shall be in accordance with EPA Method 525.2.
B.7.4.4 Phenol and minimally-substituted phenols
Analysis for phenol and minimally-substituted phenols shall be in accordance with EPA Method 420.2. Analysis for maximally-substituted phenols shall be performed by GC/MS base/acid scan (see annex B, section B.7.4.2).
B.7.5 Radionuclides analysis
Analyses for radionuclides shall be performed in accordance with Prescribed Procedures for Measurement of Radioactivity in Drinking Water, EPA-600/4-80-032. When no EPA method is provided, analyses shall be performed in accordance with Standard Methods for the of Water and Wastewater (most current edition). If neither of these two references address the required parameters and matrix, or if an alternate method is desired, method validation shall be completed prior to the application of the method (see annex B, section B.7.2.5).
B13B.7.6 RVCM analysis
B.7.6.1 General requirements for RVCM analysis
This method covers the analysis of residual vinyl chloride monomer (RVCM) in PVC and CPVC potable water products using gas chromatography. Method sensitivity is 0.5 ppm (mg/Kg) when analyzing 0.5 g of plastic material, using Flame Ionization Detector (FID).
B.7.6.2 Extraction of samples for RVCM analysis
Polyvinyl chloride (PVC) and chlorinated polyvinyl chloride (CPVC) products shall be evaluated for RVCM in the product wall. The RVCM concentration shall be determined in the wall, rather than in the extraction water, because very low levels of vinyl chloride cannot be as reliably detected in the extraction water.
B.7.6.2.1 Sample preparation for RVCM analysis
PVC and CPVC samples shall be prepared as described in the following procedure. All samples shall be prepared in duplicate.
NOTE – The weight of the sample and the duplicate should not differ by more than 0.005 g.
B.7.6.2.2 Standards for RVCM analysis
Both a standard stock solution and a secondary dilution standard shall be prepared for the RVCM analysis, using vinyl chloride gas (99.9%) and N, N-dimethylacetamide (DMAC).
B.7.6.2.3 Standard stock solution for RVCM analysis
The standard stock solution shall be prepared as follows:
(a) Pipette approximately 9.8 mL of DMAC into a 10 mL volumetric flask;
(b) Allow the flask to stand unstoppered until the wetted surface has dried;
(c) Weigh the flask and stopper to the nearest 0.1 mg and record the weight;
(d) Fill a 50 mL valved gas-tight syringe with vinyl chloride gas to the 50 mL mark;
(e) Lower the needle to 5 mm above the meniscus of the DMAC and slowly introduce the standard above the surface;
(f) Immediately reweigh the flask and contents and record the weight;
(g) Dilute to volume with DMAC, stopper, and mix;
(h) Transfer the solution into a PTFE sealed screw-cap vial;
B14(i) Store at −10°C to −20°C (14°F to −4°F); and
(j) Calculate stock standard solution with respect to a 0.500 g sample as follows:
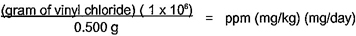
B.7.6.2.4 Secondary dilution standard for RVCM analysis
Using the stock standard solution, a secondary dilution in DMAC shall be prepared that is representative of a concentration suitable for making calibration standards and spikes.
B.7.6.3 Apparatus for RVCM analysis
The following apparatus shall be used for RVCM analysis:
– Gas Chromatograph (GC) - equipped with a mL headspace sampling system, 80°C oil bath, FID, data recording system, autosampler;
– Column: 6 foot × 2 mm ID glass column packed with 1 percent SP-1000 on Carbopack B 60/80 mesh. Equivalent columns shall be permitted as long as the column provides maximum separations from interferences and the ability to meet established accuracy and precision;
– GC Conditions: The analysis shall be performed using an over temperature program whereby the initial temperature of approximately 70°C (158°F) is held for 5 minutes, increased at 70°C/minute (158°F/minute to approximately 220°C (428°F) and held until N, N-Dimethylacetamide (DMAC) elutes (total run time about 16 minutes);
– The injector, detector, and sample loop temperature shall be held at approximately 200°C, 275°C, and 80°C, (392°F, 527°F, and 176°F) respectively;
– The helium carrier gas shall have a flow rate of 20 mL/minute. The headspace shall have a flow rate of 5 mL/minute. The hydrogen and air flows for the flame shall be approximately 30 and 400 mL/minute, respectively.
NOTE – All of these flow rates will vary somewhat between GCs to optimize separation and response. The above are given only as guidelines.
B.7.6.4 RVCM analysis
The sample and standards prepared in annex B, sections B.7.6.2.1 and B.7.6.2.2 shall be loaded into an auto sampler and equilibrated to 80°C (176°F) for 10 minutes prior to analysis.
B.7.6.5 Quality control for RVCM analysis
Duplicate analysis shall be performed on each sample. Duplicate spiked samples shall be run at the rate of one (1) set per 10 samples. An instrument standard is run with every 10 analysis (5th sample), and a reagent blank is required for each sample set. Quality control charts shall be developed and maintained and used as a check on the analytical system.
B.7.6.6 Evaluation and pass/fail criteria for RVCM analysis
PVC and CPVC products, with an RVCM concentration of less than or equal to 3.2 mg/kg, shall be considered acceptable. This acceptance criteria was determined using the equation described below:
B15Mw = 4/r [D/B]½ [(t + t0)½-t½]Mp,
where:
Mw = RVCM diffused into water (mg/L);
Mp = RVCM concentration in the PVC wall (mg/kg);
NOTE – A factor of 1.4 corrects for the ratio of density of water to PVC.
r = Pipe radius (cm);
D = Diffusivity constant,
where:
D = D0 × e(−17,000/RT);
R = Gas Constant 1.987°K−1;
T = Temperature (°K);
D0 = 3.7 cm2/sec (319680 cm2/day);
t0 = Diffusion time period (days);
t = Product age at beginning of the diffusion time (days);
30°C = 303°K.
The calculations shall be as follows:
Mw = 4/r [D/B]½ [(t + t0)½-t½](Mp × 1.4 kg/L);
Mp = 3.2 mg/kg
NOTE – the following assumptions were used in the preceding calculations:
– 30 day old product is tested equivalent to 1 inch inner pipe diameter;
– t0 = 16 hours (0.67 days);
– Mw = 0.2μg/L (0.0002 mg/L)1).
1) This concentration is based on the USEPA MCL for vinyl chloride (2 μg/L), and since the SPAC = 1/10 MCL, the SPAC = 0.2 μg/L, or 0.0002 mg/L.
B16B.7.7 Solvent analysis
This section outlines the general procedure for determining solvent levels in the extraction water. The method described below is based on direct injection gas chromatography with flame ionization detection (FID). In some instances, an enhancement step (e.g., purge and trap [cold or heated], headspace analysis) shall be required to complete the analysis. The choice of enhancement shall be dependent on the desired detection levels of the solvent of interest. The method sensitivity for direct injection is approximately 0.1 mg/L (100 ppb) for selected solvents.
B.7.7.1 General requirements for solvent-containing materials
These products shall be evaluated to determine the solvent leaching rates over time, if applicable. The relationship between contaminant concentration and time shall be determined by plotting a minimum of five points. In many instances, direct injection shall be sufficient only for the early testing period. When direct injection is no longer adequate for determining a concentration, a more sensitive method shall be required (i.e., purge and trap).
B.7.7.2 Apparatus for solvent analysis
The recommended apparatus shall be a gas chromatograph (GC) equipped with an FID, temperature programming, data recording system, and an autosampler. A purge and trap (with and without heat) system and headspace sampling system shall also be available.
NOTE – The analysis conditions may require adjustment relative to the specific solvent or solvent system being evaluated.
B.7.7.3 Quality control for solvent analysis
Duplicate matrix spike samples shall be run at the rate of one (1) set per 10 samples or less. An instrument standard shall be run with every 10 samples and a reagent blank shall be required for each daily analysis. Quality control charts shall be developed, maintained, and used as a check on the analytical system.
B.8.1 General
This section provides the calculations used to determine the level of contaminants projected “at the tap” based on the level of contaminants identified during laboratory analysis. The normalized contaminant concentration shall be compared to the requirements established in annex A.
B.8.2 Definitions
B.8.2.1 residential: Products used in buildings.
B.8.2.2 service line: Products used from the water main to building plumbing systems.
B.8.2.3 multiple user service line: Products used between the water main and multiple family residences or commercial buildings.
B.8.2.4 water main (distribution): Products used in locations other than buildings or service lines.
B.8.2.5 multiple-installation products: Products present in the drinking water system at regularly repeating intervals.
B17B.8.3 Normalization factor
To account for any differences in surface area-to-volume ratios between laboratory and actual field use conditions, an adjustment or conversion may be needed using the equation below:
NF = (N1) × (N2),
N2 = VF(static)−,
VF(flow)
Where:
SAF = Surface area exposed in the field;
SAL = Surface area exposed in the laboratory;
VL = Volume of extraction water used in the laboratory;
VF(static) = Volume of water the product is exposed to in the field for the static condition;
VF(flow) = Volume of water the product is exposed to in the field under flow conditions during a period of time equivalent to the laboratory test.
B.8.3.1 Static condition
The contaminant concentration shall be adjusted to reflect differences in surface area-to-volume relationships between laboratory and field exposures under static conditions. This calculation shall use the N1 term defined in annex B, section B.8.3. The N2 term shall always equal one when calculating normalized static concentrations.
For multiple-installation products, (e.g. pipes, fittings, and joining and sealing products used with pipes and fittings) the VF(static) component of the N1 term shall be the volume of water contained within the assumed length of pipe corresponding to the segment of the system in which the product is used (e.g., 100 feet of pipe in the service line or 280 feet of pipe in the residence).
For valves, water meters, service saddles, backflow preventers and other products not present in the system at regularly repeating intervals, the VF(static) component of the N1 term shall be the volume of water a product holds (on its own) when filled to capacity; VF(static) shall equal one L (0.264 gal) for all products that, when filled to capacity, hold (on their own) less than 1 L of water.
NOTE – Table B9 details the assumptions and resulting N1 values for typical product categories.
B.8.3.2 Flowing conditions
In addition to the static condition, the contaminant concentration shall also be adjusted to reflect differences between laboratory and field exposures under flowing conditions. For this calculation, N2 will vary depending on use. For those products not having specific flowing N2 factors outlined in table B9, product literature or operational procedures shall be consulted.
B18NOTE – Table B9 details the assumptions and resulting N2 values for typical product categories.
B.8.4 Normalization of service line and residential products
B.8.4.1 For all service line and residential products, with the exception of mechanical plumbing devices covered under section 9, a single normalized static concentration shall be determined for each contaminant.
NOTE – For residential and service line products, the static condition is the most conservative normalization since the N2 values for these products are ≤0.1.
B.8.4.2 For in-line devices, with the exception of expansion tanks and pressure tanks, the static normalized contaminant concentration shall be multiplied by an additional normalization factor, N3. The factor N3 = 1/DF, where DF is equal to the ratio of the contaminant concentration in the device to the contaminant concentration at the tap. The value of N3 for in-line devices shall be 0.33.
B.8.4.3 For all in-line devices, normalized contaminant concentrations shall be adjusted to a 12-hour exposure when the final exposure is other than 12 hours in length.
NOTE – For example, when the final exposure for an in-line device is 16 hours, the normalized contaminant concentrations shall be multiplied by a factor of 12/16.
B.8.5 Normalization for chemical feeders and generators (B.9.5 in ANSI/NSF 61-2001 deleted; this and subsequent numbers adjusted.)
Chemical feeders and generators, feeder components, and the materials used therein, present a special case because the materials are in contact with a concentrated chemical, which is then diluted at the prescribed feed rate, rather than in direct contact with water.
In addition to the equation in annex B, section B.8.3, the following normalization factor shall be used to estimate the normalized concentration of a contaminant in the finished drinking water:
NF = N1 × N2 × N4,
Where:
– VTC = Volume of concentrated treatment chemical contacted or generated by the device during a period of time equivalent to the laboratory test.
– VWT = Volume of raw water treated with the concentrated chemical when dosed at the prescribed feed rate during a period of time equivalent to the laboratory test.
B.8.6 Normalization for other products
The normalization factors described below shall be applied to products and materials not covered in annex B, sections B.8.4 and B.8.5. For these products, a single normalized concentration (either static condition or flowing condition, whichever is most conservative) shall be determined for each contaminant. For products which have a flowing N2 value ≤ 0.1, the static condition shall be the most conservative condition. For products which have a flowing N2 value > 0.1, the flowing condition shall be the most conservative condition. Normalization factors which are not included in annex B, table B9 shall be determined on a case-by-case basis using the equation in annex B, section B.8.3. Where a product is available in various sizes, the product with the highest surface area-to-volume ratio (typically the smallest diameter) shall be evaluated. For products, components, or materials that may be used in any of the 4 end use categories in annex B, table B9, qualifying by use of the largest normalization factor shall quality other use categories. Table B9 in this annex details the assumptions and resulting N1 and N2 values for various product categories.
B19B.8.7 Normalized concentration
The concentration of a contaminant in the finished drinking water shall be estimated using the following calculation.
Normalized Concentration = (Laboratory Concentration) × (Normalization Factor)
B.8.7.1 Static condition
The normalized contaminant concentration under static conditions shall be compared to the EPA MCL or the calculated TAC (as specified in annex A), and shall be less than or equal to the MCL or TAC.
B.8.7.2 Flowing condition
The normalized contaminant concentration under flowing conditions shall be compared to the Single Product Allowable Concentration (SPAC) (as specified in annex A), and shall be less than or equal to the SPAC.
B.8.7.3 Barrier materials containing solvents
Products/materials containing solvents shall be exposed such that the solvent leaching rates over time are determined. The relationship between normalized contaminant concentrations and time shall be determined and plotted with a minimum of five points. The normalized contaminant concentrations shall be compared to the STEL as specified in annex A, section A.5.
B.8.7.4 Joining and sealing materials containing solvents
The manufacturer shall have the option to initiate additional exposure testing to determine contaminant concentrations over time for solvent-containing materials. The relationship between contaminant concentrations and time shall be determined, and plotted with a minimum of 5 points. The normalized contaminant concentrations shall be calculated and then compared to the STEL as specified in annex A, section A.5.
B.8.8 Normalization for endpoint devices, components, and materials
B.8.8.1 Normalization for lead
For endpoint products, other than commercial kitchen products, each laboratory concentration shall be normalized using the equation in annex B, section B.8.3 where: VF(static) = 1 L (0.26 gal) and N2 = 1, and shall be multiplied by the cold mix volume adjustment factor (see 9.2.1).
For commercial kitchen products, each laboratory concentration shall be normalized using the equation in annex B, section B.8.3 where VF(static) = 18.9 L (5 gal) and N2 = 1 and shall be multiplied by the CMV adjustment factor (see 9.2.1).
A parametric data evaluation (annex B, section B.8.9) shall be used to evaluate the test results for lead.
When a device or component has been tested for lead through separate exposure of two or more components or materials, the values of the test statistic Q for each exposure shall be summed. The summed test statistic Q shall be evaluated against the criteria in annex B, section B.8.9.
B.8.8.2 Normalization for all analytes except lead
For endpoint products other than commercial kitchen products, the laboratory concentration shall be normalized using the equation in annex B, section B.8.3 where: VF(static) = 1 L (0.26 gal) and N2 = 1, and shall be multiplied by the CMV adjustment factor (see 9.2.1).
B20For commercial kitchen products, each laboratory concentration shall be normalized with the equation in annex B, section B.8.3 where: VF(static) = 18.9 L (5 gal) and N2 = 1, and shall be multiplied by the CMV adjustment factor (see 9.2.1).
When one sample is tested, the normalized contaminant concentrations from exposure on Day 19 shall be compared to their respective SPACs. If more than one sample is tested, the geometric mean of normalized contaminant concentrations from exposure at Day 19 shall be compared to their respective SPACs.
B.8.9 Parametric data evaluation
The term “product” connotes “endpoint devices, components, and materials”. This procedure is based on testing a sampling of products to determine the lead leaching concentrations of the product line. A derived test statistic determines whether the product line is acceptable under this standard. The calculations assume that the lead dosage leached from the product is lognormally distributed.
The number of products to be tested shall be specified by the manufacturer, though a minimum of 3 is required. It is recommended that product lines thought to be marginally acceptable (those that leach higher, but acceptable, dosages of lead) should be tested for more than the minimum number of products. For each of the products tested, the “product dosage” Di is derived from the test data as detailed in annex B, section B.8.9.2.These dosages are used to calculate the test statistic Q, which determines whether the product line is acceptable. Q is an exact 90% upper confidence bound on the 75th percentile product dosage.
In the event of a product failure, there is provision for a single retest. Retest results shall be combined with those from the initial test. The accumulated product dosages shall be used to calculated the retest statistic, R, which determines whether the product line is acceptable. R is an exact 99% upper confidence bound on the 75th percentile product dosage.
B.8.9.1 Test data
The analytical protocol described in annex B, section B.5.4 generates nine measured lead dosages (on Days 3, 4, 5, 10, 11, 12, 17, 18, and 19) leached from each of the products sampled from a particular product line. The number of products tested is defined as “n”. The test data is described as (9 × n) data values of ×ij (ith product measured on the jth day) and is shown in annex B, table B10. This data is used to calculate the product dosage Di, for each of the tested products.
This data is used to calculate the statistics Q and R for the initial test and retest, respectively.
B.8.9.2 Calculations
The test statistic depends upon the log-dosage mean and standard deviation. These values are derived as follows. Calculate the natural log-transformed value Yij = In(Xij) of the original data values. For each of the products tested, calculated the product dosage Di across the nine measured days, where:
B21Di = eYi
and
Calculate the log-dosage mean of Yi and the log-dosage standard deviation of Yi for each product, where:
and
Log-dosage standard deviation =
B.8.9.3 Initial test statistic
The test statistic, Q shall be determined as
[Illegible Text Omitted on Page B22]@[Illegible Text Omitted on Page B22]
Where the log-dosage mean, [Illegible Text Omitted on Page B22] and the log-dosage standard deviation, S, are determined using the procedures described in annex B, section B.8.9.2. The value of k1 depends upon the sample size. Table B11 in this annex presents the value of k1 for a range of sample sizes. The acceptability of the product line depends upon the value of the test statistic, where:
Case I. If Q ≤ 11 μg, the product line has tested as acceptable.
Case II. If Q > 11 μg, the product line has tested as unacceptable.
When a device or component has been tested for lead through separate exposure of two or more components or materials, the summed value of the test statistic Q shall be compared to the preceding criteria.
B.8.9.4 Retest statistic
The retest statistic, R shall be determined as:
[Illegible Text Omitted on Page B22]@[Illegible Text Omitted on Page B22]
where the log-dosage mean, [Illegible Text Omitted on Page B22] and the log-dosage standard deviation, S are determined using the procedures described In annex B, section B.8.9.2. The value of k2 depends upon the sample size. Annex B, table B12 presents the value of k2 for a range of sample sizes. The acceptability of the product line depends upon the values of the retest statistic, where:
B22Case I. If R ≤ 11 μg, the product line has tested as acceptable.
Case II. If R > 11 μg, the product line has tested as unacceptable.
B.9.1 Chemical characteristics
For extraction waters shall be available for exposure:
B.9.2 Reagents
B.9.2.1 Reagent water
Deionized water prepared to meet specifications for ASTM D 1193 Type II reagent water.
B.9.2.2 Phosphate buffer stock solutions (0.1M)
Dissolve 13.89 g sodium dihydrogen phosphate monohydrate in reagent water, dilute to 1.0 L and mix thoroughly. Prepare fresh weekly. This buffer shall be used with only the magnesium hardness reagent.
B.9.2.3 Magnesium hardness stock solution (0.04M)
Dissolve 8.13 g magnesium chloride hexahydrate in reagent water, dilute to 1.0 L, mix thoroughly. Prepare fresh weekly.
B.9.2.4 Chlorine stock solution (0.025M)
Dilute 7.3 mL reagent grade sodium hypochlorite (5% NaOCI) to 200 mL with reagent water. Store in tightly stoppered amber reagent bottle protected from light and stored at 20°C (68°F). Prepare fresh weekly.
B.9.2.4.1 Determining chlorine stock solution strength
Determine the strength of the chlorine stock solution by diluting 1.0 mL to 1.0L with reagent water. Immediately analyze for total residual chlorine. Refer to this determination as “A”.
B.9.2.4.2 Determining amount of chlorine stock solution required to obtain 2 ppm residual chlorine
To determine the volume of the chlorine stock solution necessary to add to the extraction water to obtain 2.0 mg/L chlorine residual, use the following formula:
where:
B23– A = chlorine equivalent per mL of chlorine stock solution (determined above).
– B = liters of extraction water.
B.9.2.5 Calcium hardness stock solution (0.04M)
Dissolve 4.44 g anhydrous calcium chloride in reagent water, dilute to 1.0 L, mix thoroughly. Prepare fresh weekly.
B.9.2.6 Sodium bicarbonate buffer (0.04M)
Dissolve 3.36 g sodium bicarbonate in reagent water and dilute to 1.0 L mixing thoroughly. Prepare fresh weekly.
B.9.2.7 Sodium hydroxide solution (0.1M)
Dissolve 4.0 g of sodium hydroxide in reagent water, dilute to 1.0 L and mix well.
B.9.2.8 Sodium borate solution (0.05M)
Dissolve 19.07 g of sodium borate decahydrate (Na2B4O7 . 10 H2O) in reagent water, dilute to 1.0 L and mix well.
B.9.3 pH = 5 water
Prepare pH 5 extraction water to contain 100 mg/L hardness and 2 mg/L available chlorine. Stock reagent solutions in the amounts shown in annex B, table B13, shall be diluted to the desired water volume with reagent water.
B.9.4 pH = 6.5 water
Prepare pH = 6.5 water to contain 100 mg/L hardness and 2 mg/L available chlorine. Stock reagent solutions in the amounts shown in table B13 shall be diluted to the desired water volume with reagent water. The pH shall be adjusted to pH 6.5 (± 0.5) using 0.1M HCI.
NOTE — It is recommended that the pH 6.5 water be protected from exposure to air during its formulation and use to minimize pH drift. Unused exposure water should be maintained under a nitrogen blanket, and product samples should be plugged or tightly covered to minimize exposure to air.
B.9.5 pH = 8 water (conditioning)
Prepare pH 8 conditioning water to contain 100 mg/L hardness and 2 mg/L available chlorine. Stock reagent solutions in the amounts shown in annex B, table B13, shall be diluted to the desired water volume with reagent water.
B.9.6 pH = 8 water (organic analysis)
Prepare pH 8 organic extraction water to contain 100 mg/L hardness and 0 mg/L available chlorine. Stock reagent solutions in the amounts shown in annex B, table B13 should be diluted to the desired water volume with reagent water.
B.9.7 pH = 10 water
Prepare pH 10 extraction water to contain 2 mg/L available chlorine. Stock reagent solutions in the amounts shown in annex B, table B13, shall be diluted to the desired water volume with reagent water.
B24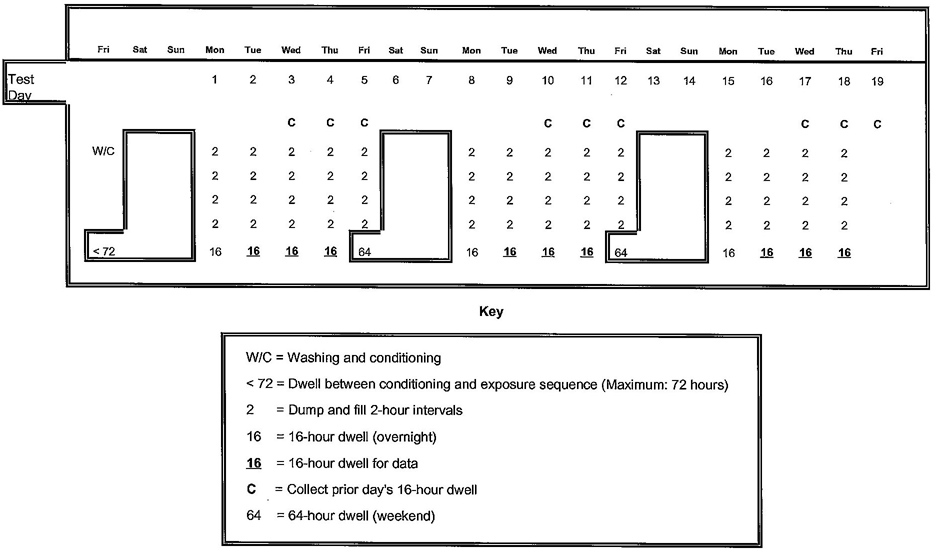
Figure B1 – Exposure sequence for mechanical plumbing devices
B25| Joining and sealing materials | Mechanical devices |
|---|---|
| Adhesives Brazing materials Fluxes Solders Caulks Gaskets Grouts Lubricants O-rings Packing Primers Sealants |
Chemical feeders Dry feeders (e.g. pellet droppers) Pressure gas injection systems Pumps Vacuum injection systems Disinfection/generators Chlorine dioxide Hypochlorite Ozone Ultraviolet Electrical wire Submersible well pumps Pumps Switches and sensors (e.g. water level, flow, pressure, temperature) Valves and related fittings (transmission/distribution system) Water process treatment devices Aeration equipment Clarifiers Electrodialysis Microfiltration Mixers Reverse osmosis Screens Strainers Ultrafiltration |
| Category | Annex B reference section | Type of samples (surface area) | Required preparation | Product exposure |
|---|---|---|---|---|
| Joining and sealing materials | 4 | 15 cm2/L | Some products applied to an appropriate substrate. Some products cut to appropriate size. Washed to remove debris accumulated during shipping and handling. |
Cold exposure = 24, 24, 24 hrs at 23°C (73°F) Hot exposure = 1, 1, 1 hr at 82°C (180°F) |
| Mechanical devices | 5 | Entire device, component, or material specimen.1) | Wash to remove debris accumulated during shipping. | Conditioning period prior to exposure (2 weeks maximum) Cold exposure = 24, 24, 24 hrs at 23°C (73°F) |
| 1)A material specimen shall be exposed using a minimum surface area-to-volume ratio or 50 cm2/L. | ||||
| Analytes of interest | pH | ||
|---|---|---|---|
| 5 | 8 | 10 | |
| Metals | X | ! | X |
| Solvents | ! | X | ! |
| Volatile organic chemicals | ! | X | ! |
NOTE – Solvents = The organic liquid component of a formulation used to dissolve solid or other liquid components of barrier materials or joining and sealing materials to facilitate their application. |
|||
| Material | Typical form |
|---|---|
| Adhesives and cements intended for joining pipe and fittings | Applied to assembled pipe and fitting joints |
| Adhesives and cements not intended for joining pipe and fittings | Applied to glass panels |
| Caulks, greases, lubricants, sealants | Applied to glass panels |
| Flux | Applied to copper sheet and heated |
| Gasket materials | ASTM D3182 tensile sheets or finished product |
| Solders and solder/flux combinations | Product heated in ceramic combustion boats |
| Time | Temperature | Comment |
|---|---|---|
| 1 hour ± 5 min | 82 ± 0.5°C (180 ± 1°F) |
Decant, discard, refill |
| 1 hour ± 5 min | 82 ± 0.5°C (180 ± 1°F) |
Decant, discard, expose as shown in annex B, section B.3.5 |
| Product | In-the-product | In-a-vessel | Other |
|---|---|---|---|
| Aeration equipment | × | Material exposed in a vessel | |
| Chemical feeders | × | Material exposed in a vessel | |
| Clarifiers | Material exposed in a vessel | ||
| Disinfection equipment | Material exposed in a vessel | ||
| Electrical wire | × | ||
| In-line devices | × | ||
| Membranes/cartridges | × | ||
| Mixers | Materials exposed in a vessel | ||
| Pumps | × | ||
| Reverse osmosis | |||
| Screens | × | ||
| Strainers | × | ||
| Switches/sensors | × | ||
| Valves | × | ||
| 1) For the purposes of this table, product may represent either the entire device or a component. These are the typical exposure conditions. However, products may be exposed in any fashion provided the exposure is consistent with requirements in annex B, section B.2. | |||
| Temperature | In-line device exposure time | Elapsed time1) in-line devices | Other mechanical devices exposure time | Elapsed time other mechanical devices1) |
|---|---|---|---|---|
| 23 ± 2°C (73 ± 4°F) | 24 hours | 24 hours | 24 hours | 24 hours |
| 23 ± 2°C (7F ± 4°F) | 24 hours | 48 hours | 24 hours | 48 hours |
| 23 ± 2°C (73 ± 4°F) | 12 to 16 hours | 60 to 64 hours | 24 hours | 72 hours |
| 1)Elapsed time does not include the initial 14-day conditioning period. | ||||
| Contaminant | Preservative | Container | Storage |
|---|---|---|---|
| Herbicide | None | 1 L (32 ounce) amber glass bottles with PTFE lid | 4°C (39°F) |
| Metals, including mercury | Conc. HNO3 to pH <2 (1.25 mL) | 125 mL (4 ounce) HDPE bottles with PTFE lid | room temp. |
| Miscellaneous organics | None | 500 mL amber bottle with PTFE lid | 4°C (39°F) |
| Pesticides | None | 500 mL (16 ounce) amber glass bottle with PTFE lid | 4°C (39°F) |
| Phenols | H2SO4 to pH <2 (2.50 mL) | 250 mL (8 ounce) (1 mL) amber glass bottle with PTFE lid | 4°C (39°F) |
| Phthalate | None | 1 L glass bottle with PTFE lid (in duplicate) | 4°C (39°F) |
| Polyaromatic hydrocarbon | None | 1 L glass bottle (in duplicate) | 4°C (39°F) |
| Radionuclides | 10.0 mL HNO3 | 1 L (32 ounce) polyethylene bottle (in duplicate) | room temp. |
| Solvents | None | 125 mL (4 ounce) amber bottle with PTFE lid | 4°C (39°F) |
| Total kjeldahl nitrogen | H2SO4 to pH <2 | 250 mL amber bottle with PTFE lid | 4°C (39°F) |
| Total organic carbon | None | 250 mL amber bottle with PTFE lid | 4°C (39°F) |
| Non-section 9 exposure for volatile organic chemicals (VOCs) | HCI | 40 mL amber glass vial with PTFE lid | 4°C (39°F) |
| Section 9 exposure for volatile organic chemicals (VOCs) | Sodium thiosulfate (a few grains to neutralize the chlorine) | 40 mL amber glass vial with PTFE lid | 4°C (39°F) |
| Product nominal diameter (n.d.) | Exposure type | Probable end-usea) | Assumptions | N1 | N2 (flowing normalization only) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n.d. > 4 in | in-the-vessel | water main | —water is exposed to the same material from the treatment plant to the service line. | calculated in accordance with annex B, section B.8.3 | 1 | |||||||||
| EXAMPLE – IN-THE-VESSEL WATER MAIN JOINING AND SEALING MATERIAL: Assumptions:
|
||||||||||||||
| n.d. = 4 in | in-the-vessel | multiple user service line | —2 use connections per service line and 180 gallons/day/user —Distance from water main to residential connections = 72 feet and therefore V5(static) = 47 gallon; flow rate equals 360 gallons per day and therefore VF(static) = 360 gallons —5 joints per multiple user service line |
calculated in accordance with annex B, section B.8.3 | 0.13 | |||||||||
| EXAMPLE— IN-THE-VESSEL MULTIPLE USER SERVICE LINE JOINING AND SEALING MATERIALS: Assumptions:
|
||||||||||||||
| 4 in > n.d. > 1 in | in-the-vessel | service line | —joining/sealing materials applied to 1 inch nominal diameter pipe —1 user connection per service line and 180 gallons/day/use —distance from water main to residence=100 feet and therefore VFC) = 4.08 gallons —flow rate equals 180 gallons per day and therefore F(flow) = 180 gallons —10 joints are present on the service line |
calculated in accordance with annex B, section B.8.3 | 0.023 | |||||||||
| EXAMPLE –IN-THE-VESSEL SERVICE LINE JOINING AND SEALING MATERIALS: Assumptions:
|
||||||||||||||
| 1 in > n.d. $ 0.5 in | in-the-vessel | residential | —joining/sealing materials applied to½ inch nominal diameter pipe —length of pipe in the residence=280 feet (140 feet cold side and 140 feet hot side) and therefore VF(static)=2.86 gallons (1.43 gallons hot and 1.43 gallons cold) —flow rate equals 180 gallons per day and therefore VF(static)=180 gallons —200 joints are present in the residential system |
calculated in accordance with annex B, section B.B.3 | 0.016 | |||||||||
| EXAMPLE –IN-THE-VESSEL RESIDENTIAL JOINING AND SEALING MATERIAL: Assumptions:
|
||||||||||||||
| n.d. ≥ 4 in | in-the-product | water main | —20 inch valves per mile (5,280 feet) —a width of 6 in is exposed for each valve |
1 | 0.002 | |||||||||
| EXAMPLE – IN-THE-PRODUCT WATER MAIN VALVE: Assumptions:
|
||||||||||||||
| 4 in > n.d.$ ½ in | in-the-product | service line or residential | —When product holds less than 1 liter (0.264 gallons) under static conditions, V F(static) =0.264 gallons=1 liter —VF(flow)=180 gallons |
calculated in accordance with annex B, section B.8.3 | 0.0015 | 0.33 | ||||||||
| EXAMPLE – IN –THE-PRODUCT SERVICE LINE VALVE: Assumptions:
be multiplied by 0.0015 to obtain the normalized flowing concentration. |
||||||||||||||
| a) Probable end-use and corresponding assumptions are related to the nominal diameter of the product | ||||||||||||||
| Product # | Measured lead dosage on day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 10 | 11 | 12 | 17 | 18 | 19 | |
| 1 | X13 | X14 | X1 | X110 | X111 | X112 | X17 | X118 | X119 |
| 2 | X23 | X24 | X25 | X210 | X211 | X212 | X217 | X218 | X219 |
| 3 | X33 | X34 | X35 | X310 | X311 | X312 | X317 | X318 | X319 |
| ! | ! | ! | ! | ! | ! | ! | ! | ! | ! |
| ! | ! | ! | ! | ! | ! | ! | ! | ! | ! |
| ! | ! | ! | ! | ! | ! | ! | ! | ! | ! |
| n | Xn 3 | Xn 4 | Xn 5 | Xn 10 | Xn 11 | Xn 12 | Xn 17 | Xn 18 | Xn 19 |
| Sample size | k1 |
|---|---|
| 3 | 2.60281 |
| 4 | 1.97224 |
| 5 | 1.69779 |
| 6 | 1.53987 |
| 7 | 1.43526 |
| 8 | 1.35984 |
| 9 | 1.30234 |
| 10 | 1.25672 |
| 11 | 1.21943 |
| 12 | 1.18824 |
| 13 | 1.16167 |
| 14 | 1.13870 |
| 15 | 1.11859 |
| 16 | 1.10080 |
| 17 | 1.08491 |
| 18 | 1.07063 |
| 19 | 1.05769 |
| 20 | 1.04590 |
| 21 | 1.03510 |
| 22 | 1.02517 |
| 23 | 1.01598 |
| 24 | 1.00747 |
| 25 | 0.99954 |
| 26 | 0.99213 |
| 27 | 0.98520 |
| 28 | 0.97869 |
| 29 | 0.97256 |
| 30 | 0.96677 |
| 31 | 0.96130 |
| 32 | 0.95612 |
| 33 | 0.95120 |
| 34 | 0.94653 |
| 35 | 0.94208 |
| 36 | 0.93783 |
| 37 | 0.93377 |
| 38 | 0.92990 |
| 39 | 0.92618 |
| 40 | 0.92262 |
| 41 | 0.91921 |
| 42 | 0.91592 |
| 43 | 0.91277 |
| 44 | 0.90973 |
| 45 | 0.90680 |
| 46 | 0.90397 |
| 47 | 0.90125 |
| 48 | 0.89861 |
| 49 | 0.89607 |
| 50 | 0.89361 |
| Sample size | k2 |
|---|---|
| 6 | 2.84809 |
| 7 | 2.49072 |
| 8 | 2.25337 |
| 9 | 2.08314 |
| 10 | 1.95433 |
| 11 | 1.85297 |
| 12 | 1.77079 |
| 13 | 1.70259 |
| 14 | 1.64491 |
| 15 | 1.59536 |
| 16 | 1.55224 |
| 17 | 1.51431 |
| 18 | 1.48063 |
| 19 | 1.45048 |
| 20 | 1.42329 |
| 21 | 1.39862 |
| 22 | 1.37611 |
| 23 | 1.35548 |
| 24 | 1.33647 |
| 25 | 1.31889 |
| 26 | 1.30257 |
| 27 | 1.28738 |
| 28 | 1.27319 |
| 29 | 1.25989 |
| 30 | 1.24740 |
| 31 | 1.23565 |
| 32 | 1.22455 |
| 33 | 1.21407 |
| 34 | 1.20413 |
| 35 | 1.19470 |
| 36 | 1.18574 |
| 37 | 1.17721 |
| 38 | 1.16907 |
| 39 | 1.16130 |
| 40 | 1.15387 |
| 41 | 1.14676 |
| 42 | 1.13994 |
| 43 | 1.13340 |
| 44 | 1.12711 |
| 45 | 1.12107 |
| 46 | 1.11526 |
| 47 | 1.10966 |
| 48 | 1.10425 |
| 49 | 1.09904 |
| 50 | 1.09401 |
| pH | Solution #1 | Solution #2 | Chlorine stock solution |
|---|---|---|---|
| 5 | 25 ml of 0.1M NaH2PO4 | 25 ml of 0.04M MgCl2 | annex B, section B.9.2.4 |
| 6.5 | 25 ml of 0.04M NaHCO3 | 25 ml of 0.04M CaCl2 | annex B, section B.9.2.4 |
| 8 (conditioning) | 25 ml of 0.04M NaHCO3 | 25 ml of 0.04M CaCl2 | annex B, section B.9.2.4 |
| 8 (organic) | 25 ml of 0.04M NaHCO3 | 25 ml of 0.04M CaCl2 | none |
| 10 | 50 ml of 0.1 NaOH | 50 ml of 0.05M Na2B4O7 | annex B, section B.9.2.4 |
This annex defines the evaluation process for materials that have been submitted for qualification as acceptable materials.
A material that has a standard material formulation or specification (e.g. ASTM), has undergone extraction testing which demonstrates that the material does not contribute any contaminant in excess of its acceptable level as determined by this Standard (see annex C, section C.3), and is accompanied by adequate documentation (section 3.4.2), shall be designated as an “acceptable material” in table C1.
Thirty randomly selected samples from a variety of manufacturers of the material, in a specific form (e.g., pipe or tube), shall undergo extraction testing. All of the samples shall have been manufactured using the same production process. Selection of analytical testing shall be performed in accordance with section 3.3. The samples shall be exposed at the maximum surface area to volume ratio for which acceptance is being sought. Depending on the specific form of the material, the samples shall be evaluated with the extraction protocol, normalization formulas and assumptions, and evaluation criteria contained in the applicable sections of this Standard.
The material's evaluation shall be supported by the following documentation:
— the published material formulation or specification to which the material is fabricated;
— literature that comprehensively addresses the production process, raw material sources, and all other factors that could potentially affect the composition and variability of the material; and
— information and data that summarize the results from the laboratory extraction of the 30 randomly selected samples, including data from a detection limit study, quality control data run concurrently with the samples, a description of the methods and instrumentation used, and a verification that the laboratory in which the extraction testing was conducted is certified for drinking water analysis by the regulatory agency having authority.
A final report that outlines the manner in which these requirements have been met shall be prepared.
C1| Material | Specific designation | Standard (product) reference | Surface area to volume ratio | End use temperature | Composition |
|---|---|---|---|---|---|
| Stainless steel | UNS S30400 (Type 304) | ASTM A 312 ASTM A 269 | 3,484 cm2/L (540 in2/L) | 30EC (86 EF) | Percent Composition: Carbon (0.08 max.), Manganese (2.00 max.), Phosphorus (0.040 max.), Sulfur (0.030 max.), Silicon (0.75 max.), Nickel (8.00–11.0), Chromium (18.0-20.0), Iron (Balance) |
| Stainless steel | UNS S30403 (Type 304L) | ASTM A 312 ASTM A 269 | 3,484 cm2/L (540 in2/L) | 30EC (86EF) | Percent Composition: Carbon (0.035 max.), Manganese (2.00 max.), Phosphorus (0.040 max.), Sulfur (0.030 max.), Silicon (0.75 max.), Nickel (8.00–13.0), Chromium (18.0-20.0), Iron (Balance) |
| Stainless steel | UNS S31600 (Type 316) | ASTM A 312 ASTM A 269 | 3,484 cm2/L (540 in2/L) | 30EC (86EF) | Percent Composition: Carbon (0.08 max.), Manganese (2.00 max.), Phosphorus (0.040 max.), Sulfur (0.030 max.), Silicon (0.75 max.), Nickel (11.00–14.0), Chromium (16.0–18.0), Molybdenum (2.0–3.0), Iron (Balance) |
| Stainless steel | UNS S31603 (Type 316L) | ASTM A 312 ASTM A 269 | 3,484 cm2/L (540 in2/L) | 30EC (86EF) | Percent Composition: Carbon (0.035 max.), Manganese (2.00 max.), Phosphorus (0.040 max.), Sulfur (0.030 max.), Silicon (0.75 max.), Nickel (10.0–15.0), Chromium (16.0–18.0), Molybdenum (2.0–3.0), Iron (Balance) |
The drinking water criteria in this annex shall be used as normative evaluation criteria for the determination of product compliance with the health effects requirements of this Standard. The values in these tables include the consensus USEPA and Health Canada drinking water criteria for contaminants evaluated by these two agencies. They also include criteria for non-regulated contaminants that have been developed according to the toxicity data requirements of annex A, and that have been externally peer-reviewed. Non-regulatory USEPA guidance values that have been reviewed and found to satisfy annex A toxicity data requirements are also included, as well as chemicals that have been evaluated using the threshold of evaluation approach.
The drinking water criteria in this annex have not been evaluated for taste and odor considerations at the concentration limits indicated.
The substances listed in annex E, tables E1, E2, E3, and E4 are not intended to encompass all of the potential analytes of interest that need to be considered when evaluating products to the requirements of this Standard. The user is cautioned that each product may have formulation dependent analytes of interest for which acceptable concentration limits have not been determined. In these cases, the user is required to develop acceptable concentration limits based on the requirements of annex A of NSF/ANSI 61 in order to determine full compliance with the Standard.
These tables are specific to NSF/ANSI 61. While the tables may be used for evaluation of impurities in drinking water treatment chemicals, the substances listed in these tables may not have been evaluated for use as direct additive drinking water treatment chemicals under NSF/ANSI 60. Use as direct additive drinking water treatment chemicals may require the consideration of different exposure parameters than those used for NSF/ANSI 61 evaluation.
Table E1 contains drinking water criteria for contaminants regulated by the USEPA and established by Health Canada. Values for each contaminant have been agreed upon by representatives of both agencies for the purpose of evaluating products against the health effects requirements of NSF/ANSI 61. For each substance, the values in the table represent a consensus decision regarding the selection of the most appropriate assessment upon which to base NSF/ANSI 61 evaluation.
At the time of publication, the indicated values were valid. These values are subject to change, however, and the user is encouraged to contact USEPA or Health Canada for the most current values. Some of these values have been developed using a linear multistage model to predict theoretical excess carcinogenic risk at low exposure concentrations. Where the database is sufficient and the compound mode of action can be determined, the USEPA is replacing the default linear multistage model with either a biologically-based cell kinetic multistage model or a margin of exposure analysis. Cancer potency (q1*) values developed using the linear multistage model may be re-evaluated in the future.
1)This annex was annex E in ANSI/NSF 61 – 2000. Annex D in ANSI/NSF 61 – 2000 was deleted because it was not necessary as a to reference annex a.
D1Table D2 contains drinking water criteria for unregulated substances for which NSF international has determined Total Allowable Concentrations (TAC) and Single Product Allowable Concentrations (SPAC) in accordance with annex A of this Standard. These criteria have been externally peer-reviewed.
At the time of publication, the indicated values were valid. These values are subject to change, however, and the user is encouraged to contact NSF International for the most current values.
Table D3 contains drinking water criteria for unregulated contaminants for which the acceptable drinking water concentrations are based on USEPA guidance values, including those in the USEPA Health Advisory and Integrated Risk Information System (IRIS) databases. A relative source contribution factor has been applied to calculation of the drinking water criteria when a relative source contribution factor was not applied as part of the USEPA risk assessment. In the absence of sufficient information to determine a data-derived relative source contribution factor, a default 20% drinking water contribution is assumed.
At the time of publication, the indicated values were valid. These values are subject to change, however, and the user is encouraged to contact USEPA for the most current values. Some of these values have been developed using a linear multistage model to predict risk at low exposure concentrations and may be re-evaluated in the future.
Table D4 contains the list of chemicals which have been evaluated under the threshold of evaluation, due to the lack of the minimum data to determine chemical specific concentrations in accordance with the requirements of annex A (see annex A, section A.2.7.1). Qualification to the threshold of evaluation category includes a comprehensive literature search for the particular substance and consideration of structure-activity relationships.
D2| Contaminant (Reference)1) | Drinking water regulatory level (MCL/MAC) (mg/L) | Single product allowable concentration (SPAC) (mg/L) |
|---|---|---|
| Organics/Pesticides | ||
| Alachlor (40 CFR §141.61, §141.61) |
0.002 | 0.0002 |
| Aldicarb Aldicarb sulphone Aldicarb sulphoxide (40 CFR §141.60, §141.61) |
0.007 (draft) |
0.0007 |
| Atrazine and metabolites Issue date: 04/93 |
0.005 | 0.0005 |
| Azinphos-methyl Issue date: 02/86 |
0.02 | 0.002 |
| Bendiocarb Issue date: 02/86 |
0.04 | 0.004 |
| Benzene (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| Benzo(a)pyrene (PAH) (40 CFR §141.60, §141.61) |
0.0002 | 0.00002 |
| Bromodichloromethane — See Trihalomethanes (total) |
N/A | N/A |
| Bromoform — See Trihalomethanes (total) |
N/A | N/A |
| Bromoxynil Issue date: 03/87 |
0.005 | 0.0005 |
| Carbofuran (40 CFR §141.60, §141.61) |
0.04 | 0.004 |
| Carbon tetrachloride (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| Chlordane (40 CFR §141.60, §141.61) |
0.002 | 0.0002 |
| Chlorodibromomethane — See Trihalomethanes (total) |
N/A | N/A |
| Chloroform — See Trihalomethanes (total) |
N/A | N/A |
| 2,4–D (40 CFR §141.60, §141.61) |
0.07 | 0.007 |
| Dalapon (40 CFR §141.60, §141.61) |
0.2 | 0.02 D3 |
| Dibromochloropropane (DBCP) (40 CFR §141.60, §141.61) |
0.0002 | 0.00002 |
| Dichlorobenzene o- (40 CFR §141.60, §141.61) |
0.6 | 0.06 |
| Dichlorobenzene m- (See o-Dichlorobenzene) |
0.6 | 0.06 |
| Dichlorobenzene p- (40 CFR §141.60, §141.61) |
0.075 | 0.0075 |
| Dichloroethane (1,2-) (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| Dichloroethylene (1,1-) (40 CFR §141.60, §141.61) |
0.007 | 0.0007 |
| Dichloroethylene (cis-1,2-) (40 CFR §141.60, §141.61) |
0.07 | 0.007 |
| Dichloroethylene (trans-1,2) (40 CFR §141.60, §141.61) |
0.1 | 0.01 |
| Dichloromethane (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| Dichloropropane (1,2-) (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| Diclofop-methyl Issue date: 03/87 |
0.009 | 0.0009 |
| Di(2-ethylhexyl)adipate (40 CFR §141.60, §141.61) |
0.4 | 0.04 |
| Di(2-ethylhexyl)phthalate (PAE) (40 CFR §141.60, §141.61) |
0.006 | 0.0006 |
| Dimethoate Issue date: 02/86 |
0.020 | 0.002 |
| Dinoseb (40 CFR §141.60, §141.61) |
0.007 | 0.0007 |
| Diquat (40 CFR §141.60, §141.61) |
0.02 | 0.002 |
| Endothall (40 CFR §141.60, §141.61) |
0.1 | 0.01 D4 |
| Endrin (40 CFR §141.60, §141.61) |
0.002 | 0.0002 |
| Ethylbenzene (40 CFR §141.60, §141.61) |
0.7 | 0.07 |
| Ethylene dibromide (EDB) (40 CFR §141.60, §141.61) |
0.00005 | 0.000005 |
| Glyphosate (40 CFR §141.60, §141.61) |
0.7 | 0.07 |
| Heptachlor (40 CFR §141.60, §141.61) |
0.0004 | 0.00004 |
| Heptachlor epoxide (40 CFR §141.60, §141.61) |
0.0002 | 0.00002 |
| Hexachlorobenzene (40 CFR §141.60, §141.61) |
0.001 | 0.0001 |
| Hexachlorocyclopentadiene (40 CFR §141.60, §141.61) |
0.05 | 0.005 |
| Lindane (40 CFR §141.60, §141.61) |
0.0002 | 0.00002 |
| Methoxychlor (40 CFR §141.60, §141.61) |
0.04 | 0.004 |
| Metolachlor Issue date: 02/86 |
0.05 | 0.005 |
| Monochlorobenzene (40 CFR §141.60, §141.61) |
0.1 | 0.01 |
| Nitrilotriacetic acid Issue date: 01/90 |
0.4 | 0.04 |
| Oxamyl (Vydate) (40 CFR §141.60, §141.61) |
0.2 | 0.02 |
| Parathion Issue date: 02/86 |
0.05 | 0.005 |
| Pentachlorophenol (40 CFR §141.60, §141.61) |
0.001 | 0.0001 |
| Phorate Issue date: 02/86 |
0.002 | 0.0002 D5 |
| Picloram (Issue date 06/88) |
0.19 | 0.019 |
| Polychlorinated biphenyls (PCB) (40 CFR §141.60, §141.61) |
0.0005 | 0.00005 |
| Simazine (40 CFR §141.60, §141.61) |
0.004 | 0.0004 |
| Styrene (40 CFR §141.60, §141.61) |
0.1 | 0.01 |
| 2,3,7,8-TCDD (Dioxin) (40 CFR §141.60, §141.61) |
3E-08 | 3E-09 |
| Tetrachloroethylene (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| 2,3,4,6-Tetrachlorophenol Issue date: 02/87 |
0.1 | 0.01 |
| Toluene (40 CFR §141.60, §141.61) |
1 | 0.1 |
| Toxaphene (40 CFR §141.60, §141.61) |
0.003 | 0.0003 |
| 2,4,5-TP (40 CFR §141.60, §141.61) |
0.05 | 0.005 |
| Trichlorobenzene (1,2,4-) (40 CFR §141.60, §141.61) |
0.07 | 0.007 |
| Trichloroethane (1,1,1-) (40 CFR §141.60, §141.61) |
0.2 | 0.02 |
| Trichloroethane (1,1,2-) (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| Trichloroethylene (40 CFR §141.60, §141.61) |
0.005 | 0.0005 |
| 2,4,6-Trichlorophenol Issue date: 02/87 |
0.005 | 0.0005 |
| Trihalomethanes (total) | 0.08 | 0.008 |
| bromodichloromethane | — | — |
| bromoform | — | — |
| chlorodibromomethane | — | — |
| chloroform (40 CFR §141.64) |
— | — D6 |
| Vinyl chloride (40 CFR §141.60, §141.61) |
0.002 | 0.0002 |
| Xylenes (total) (40 CFR §141.60, §141.61) |
10 | 1 |
| Regulated Metals | ||
| Antimony (40 CFR §141.60, §141.62) |
0.006 | 0.0006 |
| Arsenic Issue date: 02/89 |
0.025 | 0.0025 |
| Barium (40 CFR §141.60, §141.62) |
2 | 0.2 |
| Beryllium (40 CFR §141.60, §141.62) |
0.004 | 0.0004 |
| Cadmium (40 CFR §141.60, §141.62) |
0.005 | 0.0005 |
| Chromium (total) (40 CFR §141.60, §141.62) |
0.1 | 0.01 |
| Copper (40 CFR §141.80; 65 FR 1950) |
TT2) (action level 1.3 mg/L) | 0.13 |
| Lead (at tap) (40 CFR §141.80; 65 FR 1950) |
TT2)3) (action level 0.015 mg/L) | 0.0015 |
| Mercury (inorganic) (40 CFR §141.60, §141.62) |
0.002 | 0.0002 |
| Selenium (40 CFR §141.60, §141.62) |
0.05 | 0.005 |
| Thallium (40 CFR §141.60, §141.62) |
0.002 | 0.0002 |
| Other Inorganics | ||
| Asbestos (40 CFR §141.60, §141.62) |
74) MFL | 0.7 MFL |
| Bromate (40 CFR §141.64) |
0.01 | 0.001 |
| Chloramines (total as CI2) (40 CFR § 141.65) |
45) | 0.4 |
| Chlorine (free as CI2) (40 CFR § 141.65) |
45) | 0.4 D7 |
| Chlorine dioxide (as CIO2) (40 CFR §141.65) |
0.85) | 0.08 |
| Chlorite (40 CFR §141.64) |
1 | 0.1 |
| Cyanide (as free cyanide) (40 CFR §141.60, §141.62) |
0.2 | 0.02 |
| Fluoride (40 CFR §141.60, §141.62) |
4 | 0.4 |
| Haloacetic acids (total) (40 CFR §141.64) |
0.06 | 0.006 |
| Nitrate (as N) (40 CFR §141.60, §141.62) |
10 | 1 |
| Nitrate (as N) (40 CFR §141.60, §141.62) |
1 | 0.1 |
| Nitrate + Nitrite (both as N) (40 CFR §141.60, §141.62) |
10 | 1 |
| Radionuclides | ||
| Beta particle and photon activity (40 CFR §141.16) |
4 mrem/y | 4 mrem/y |
| Gross alpha particle activity (40 CFR §141.15) |
15 pCi/L | 15 pCi/L |
| Combined Radium 226 and 228 (40 CFR §141.15) |
5 pCi/L | 5 pCi/L |
| 1) The references for criteria based on U.S. primary drinking water regulations are from the U.S. Code of Federal Regulations, Title 40 (Protection of Environment), revised as of July 1, 1999. This document is available on-line at www.access.po.gov/cgi-bin/cfrassemble.cgi. Issue dates are given for criteria based on Health Canada guidelines. Additional information on the guidelines for these chemicals is available at www.hc-sc.gc.ca/water quality. 2) TT = Treatment technique. 3) For Section 9 products, a Q statistic value of 11 µg lead for a 1 liter draw is used as the evaluation criteria. This is based on the assumption that sources other than the Section 9 device contribute 4 µg for a 1 liter draw, resulting in a total limit of 15 µg lead for a 1 liter draw. 4) MFL = Million Fibers per Liter, with fiber length > 10 microns. 5) Value represents the maximum residual disinfectant level (MRDL). |
||
| Substance | CAS # | Total allowable concentration (TAC)1) mg/L | Single product allowable concentration (SPAC)1) mg/L | Source of supporting documentation |
|---|---|---|---|---|
| Acetophenone | 98-86-2 | 0.051 | 0.05 | NSF action level2) External peer review date: 08/06/98 |
| Benzaldehyde | 100-52-7 | 0.9 | 0.09 | NSF action level2) External peer review date: 04/15/99 |
| Furfural | 98-01-1 | 0.081) | 0.08 | NSF action level2) External peer review date: 08/06/98 |
| Isopropylbenzene (Cumene) | 98-82-8 | 4.0 | 0.4 | NSF action level2) (Derived from the oral RfD on the EPA IRIS database)3) External peer review date: 04/15/99 |
| Melamine | 108-78-1 | 3.0 | 0.3 | NSF action level2) External peer review date: 04/14/99 |
| Methyl isoamyl ketone (MIAK) | 110-12-3 | 0.051 | 0.05 | NSF action level2) External peer review date: 08/06/98 |
| 1) The TAC and the SPAC are equal because of data limitations that prevent the TAC from being set at a higher concentration. 2) NSF action levels have been derived according to the requirements of NSF/ANSI 61, annex A. 3) Data published subsequent to publication of the IRIS file have been reviewed by NSF, and found to support the IRIS-derived concentrations. |
||||
| Substance | CAS # | Total allowable concentration (TAC) mg/L | Single product allowable concentration (SPAC) mg/L | Source of supporting documentation1) 2) |
|---|---|---|---|---|
| Inorganics | ||||
| Manganese | 7439-96-5 | 0.3 | 0.03 | Derived from the oral RfD on the EPA IRIS database, with a 3x modifying factor because of the large contribution from food sources and a default 20% relative source contribution for drinking water Verification date: 05/12/95 |
| Molybdenum | 7439-98-7 | 0.04 | 0.004 | EPA Lifetime Drinking Water Health Advisory Issue date: 1990 |
| Organics | ||||
| Acrylamide | 79-06-1 | 0.0001 | 0.00001 | EPA Drinking Water Health Advisory 10−5/10−6 cancer risk levels Issue date: 1987 |
| Acrylic acid | 79-10-7 | 4 | 0.4 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 02/17/94 |
| Acrylonitrile | 107-13-1 | 0.0006 | 0.00006 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 02/11/87 |
| Aniline | 62-53-3 | 0.06 | 0.006 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 06/03/87 |
| Azobenzene | 103-33-3 | 0.003 | 0.0003 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 02/03/88 |
| Benzidine | 92-87-5 | 0.000002 | 0.0000002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 12/17/86 D10 |
| Benzoic acid | 65-85-0 | 30 | 3 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 09/17/87 |
| Benzotrichloride | 98-07-7 | 0.00003 | 0.000003 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 08/02/89 |
| Benzyl chloride | 100-44-7 | 0.002 | 0.0002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 03/01/89 |
| Bis(chloroethyl)ether | 111-44-4 | 0.0003 | 0.00003 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 07/23/86 |
| Bis(chloromethyl)ether | 542-88-1 | 0.000002 | 0.0000002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 05/04/88 |
| Bromochloromethane | 74-97-5 | 0.01 | 0.001 | EPA Lifetime Drinking Water Health Advisory Issue date: 1989 |
| Caprolactam | 105-60-2 | 4 | 0.4 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 03/24/88 |
| Chlorobenzilate | 510-15-6 | 0.1 | 0.01 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 05/17/89 |
| Cyclohexylamine | 108-91-8 | 1 | 0.1 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 09/17/87 D11 |
| 3,3′ -Dichlorobenzidine | 91-94-1 | 0.0008 | 0.00008 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 11/30/88 |
| p,p′ -Dichlorodiphenyl Dichloroethane (DDD) | 72-54-8 | 0.001 | 0.0001 | EPA IRIS 10−5/10−6 cancer risk levels. erification date: 06/24/87 |
| p,p′ -Dichlorodiphenyl Dichloroethylene (DDE) | 72-55-9 | 0.001 | 0.0001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 06/24/87 |
| p,p′ -Dichlorodiphenyl Trichloroethane (DDT) | 50-29-3 | 0.001 | 0.0001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 06/24/87 |
| 1,3-Dichloropropene mixed isomers cis- trans- |
542-75-6 10061-01-5 10061-02-6 |
0.002 | 0.0002 | EPA Health Advisory 10−5/10−6 cancer risk levels Issue date: 1988 |
| 2,6-Dimethylphenol | 576-26-1 | 0.004 | 0.0004 | Derived from the oral RfD on the EPA IRIS database with an default 20% relative source contribution for drinking water. Verification date: 01/22/86 |
| 3,4-Dimethylphenol | 95-65-8 | 0.007 | 0.0007 | Derived from the oral RfD on the EPA IRIS database with an default 20% relative source contribution for drinking water. Verification date: 01/22/86 |
| 2,4/2,6-Dinitrotoluene mixture | Not Applicable | 0.0005 | 0.0005 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 05/03/89 |
| 1,4-Dioxane | 123-91-1 | 0.07 | 0.007 | EPA Drinking Water Health Advisory 10−5/10−6 cancer risk levels Issue date: 1987 |
| 1,2-Diphenylhydrazine | 122-66-7 | 0.0005 | 0.00005 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 10/29/86 D12 |
| Epichlorohydrin | 106-89-8 | 0.04 | 0.004 | EPA Drinking Water Health Advisory 10−5/10−6 cancer risk levels Issue date: 1987 |
| Ethylene glycol | 107-21-1 | 10 | 1 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 03/19/87 |
| Ethylene thiourea | 96-45-7 | 0.0006 | 0.00006 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 02/20/91 |
| Formaldehyde | 50-00-0 | 1 | 0.1 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 06/20/90 |
| Hydrazine/Hydrazine sulfate | 302-01-2 | 0.0001 | 0.00001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 06/03/87 |
| Isophorone | 78-59-1 | 0.4 | 0.04 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 08/05/92 |
| Maleic anhydride | 108-31-6 | 0.7 | 0.07 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 03/24/88 |
| 4,4′-Methylene bis (N,N′-dimethyl)aniline | 101-61-1 | 0.008 | 0.0008 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 04/05/89 D13 |
| Nitrobenzene | 98-95-3 | 0.004 | 0.0004 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 07/08/85 |
| N-Nitroso-di-n-butylamine | 924-16-3 | 0.00006 | 0.000006 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 10/29/86 |
| N-Nitroso-N-methylethylamine | 10595-95-6 | 0.00002 | 0.000002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 02/11/87 |
| N-Nitroso-di-N-propylamine | 621-64-7 | 0.00005 | 0.000005 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 02/11/87 |
| N-Nitroso-di-N-propylamine | 621-64-7 | 0.00005 | 0.000005 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 02/11/87 |
| N-Nitrosodiethanolamine | 1116-54-7 | 0.0001 | 0.00001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 01/28/87 |
| N-Nitrosodiethylamine | 55-18-5 | 0.000002 | 0.0000002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 10/29/86 |
| N-Nitrosodimethylamine | 62-75-9 | 0.000007 | 0.0000007 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 10/29/86 |
| N-Nitrosodiphenylamine | 86-30-6 | 0.07 | 0.007 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 02/11/87 |
| N-Nitrosopyrrolidine | 930-55-2 | 0.0002 | 0.00002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 10/14/86 D14 |
| Propargite | 2312-35-8 | 0.1 | 0.01 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Verification date: 03/23/88 |
| Propylene oxide | 75-56-9 | 0.001 | 0.0001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 04/05/90 |
| 1,1,1,2-Tetrachloroethane | 630-20-6 | 0.01 | 0.001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 05/04/88 |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 0.002 | 0.0002 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 06/26/86 |
| 1,2,3-Trichloropropane | 96-18-4 | 0.05 | 0.005 | EPA Health Advisory 10−5/10−6 cancer risk levels Issue date: 1989 |
| 2,4,6-Trinitrotoluene | 118-96-7 | 0.01 | 0.001 | EPA IRIS 10−5/10−6 cancer risk levels. Verification date: 09/22/88 |
1) Criteria are derived from the oral RfD on the EPA IRIS database as follows:
Where: 70 kg = assumed adult body weight 2 L/day = assumed adult water consumption relative source contribution factor = percentage of daily exposure to the substance represented by drinking water (default value is 20%) Other criteria have been used directly, unless otherwise noted. 2) The IRIS verification date represents the date the oral RfD or the cancer risk assessment was peer reviewed by the EPA. Refer to the online IRIS database for the complete update and revision history of the IRIS files: (http://www.epa.gov/ngispgm3/iris/subst). |
||||
| Substance | CAS # |
|---|---|
| Inorganics | |
| Yttrium | 007440-65-5 |
| Organics | |
| Acenaphthylene | 000208-96-8 |
| Acetophenone, p-isopropyl- | 000645-13-6 |
| Acetophenone, 2's-methyl- | 000577-16-2 |
| Acetophenone, 4-methyl | 000122-00-9 |
| Acetophenone, alpha-hydroxy- | 000582-24-1 |
| Acetophenone, 3'-methyl- | 000585-74-0 |
| Acetophenone, 4'-isopropenyl | 005359-04-6 |
| Acetophenone, 4's-hydroxy- | 000099-93-4 |
| Alcohols, C12-C15, ethoxylated propoxylated | 068551-13-3 |
| Allyl phenol ether | 001746-13-0 |
| Aminoundecanoic acid, 12- | 000693-57-2 |
| Benzaldehyde, 3,5-di-tert-butyl-4-hydroxy- | 001620-98-0 |
| Benzaldehyde, 4-hydroxy-3-methoxy (Vanillin) | 000121-33-5 |
| Benzaldehyde, 3,5-dimethoxy-4-hydroxy- | 000134-96-3 |
| Benzaldehyde, 2-hydroxy- | 000090-02-8 |
| Benzaldehyde, 2-hydroxy-4-methoxy | 000673-22-3 |
| Benzaldehyde, tert-butylmethyl- | 066949-23-3 |
| Benzene, 2-ethoxyethenyl- | 017655-74-2 |
| Benzene, (2-methoxy-1-methylethyl)- | 065738-46-7 |
| Benzene, divinyl- | 001321-74-0 |
| Benzene, (1-methoxy-1-methylethyl)- | 000935-67-1 |
| Benzene, 1,1-oxybis- | 000101-84-8 |
| Benzene, 1,3-Dimethyl-5-isopropyl- | 004706-90-5 |
| Benzene, 4,6-diisopropyl-1,3-dimethyl- | 005186-68-5 |
| Benzeneacetaldehyde | 000122-78-1 |
| Benzenediamine, ar,ar-diethyl-ar-methyl | 068479-98-1 |
| Benzenedimethanol, a,a,a',a'-tetramethyl-1,4- | 002948-46-1 |
| Benzenedimethanol, a,a,a', a'-tetramethyl-1,3- | 001999-85-5 |
| Benzenemethanamine, N-(phenylmethylene)- | 000780-25-6 |
| Benzenemethanol, 4-(1-methylethyl)- | 000536-60-7 |
| Benzenepropanoic acid, 3,5-bis(1, 1-dimethylethyl)-4-hydroxy- | 020170-32-5 D16 |
| Benzenesulfonamide, 4-methyl- | 000070-55-3 |
| Benzenesulfonyl isocyanate, 4-methyl | 004083-64-1 |
| Benzenetricarboxylic acid, 1,2,4- | 000528-44-9 |
| Benzofuran, methyl- | 025586-38-3 |
| Benzoic acid, m-methyl- | 000099-04-7 |
| Benzoic acid, 4-tert-butyl- | 000098-73-7 |
| Benzoic acid, ethyl ester | 000093-89-0 |
| Benzoic acid, methyl ester | 000093-58-3 |
| Benzonitrile | 000100-47-0 |
| Benzoquinone, 2,6-di-t-butyl- | 000719-22-2 |
| Benzoquinone, 2,5-di-tert-pentyl-p- | 004584-63-8 |
| Benzothiazole | 000095-16-9 |
| Benzothiazole, ethylamino- | 028291-69-2 |
| Benzothiazole, 2-(methylmercapto)- | 000615-22-5 |
| Benzothiazole, 2-methyl- | 000120-75-2 |
| Benzothiazole, 2-(morpholinothio)- | 000102-77-2 |
| Benzothiazole-2-thione, N-methyl- | 002254-94-6 |
| Benzotriazole, 2-(2-hydroxy-5-methyl-phenyl)- | 002440-22-4 |
| 2-Benzothiazolinone | 000934-34-9 |
| Benzyl ethyl ether | 000539-30-0 |
| Benzyl alcohol, 4-ethoxy | 006214-44-4 |
| Benzyl alcohol, alpha, alpha, 4-trimethyl- | 001197-01-9 |
| Benzyl alcohol, a,a-dimethyl-p-isopropyl- | 003445-42-9 |
| Benzylamine | 000100-46-9 |
| Benzyldiphenylphosphine oxide | 002959-74-2 |
| Binaphthyl sulfone | 032390-26-4 |
| Borneol | 000507-70-0 |
| 1-Butanamine,N,N-dibutyl- | 000102-82-9 |
| Butanedioic acid | 000110-15-6 |
| Butanediol dimethacrylate, 1,4- | 002082-81-7 |
| Butanetricarboxylic acid, 2-phosphono-, 1,2,4- | 037971-36-1 |
| Butanoic acid | 000107-96-2 |
| Butanoic acid, 3,3-dimethyl- | 001070-83-3 |
| Buten-1-ol, 2-methyl-2- | 004675-87-0 D17 |
| Buten-1-ol, 3-methyl-3- | 000763-32-6 |
| Butenal, methyl- | 001115-11-3 |
| Butene, 2,3-dichloro-2-methyl- | 000507-45-9 |
| Butenoic acid, 2- | 003724-65-0 |
| Butenoic acid, 3- | 000625-38-7 |
| Butyl isocyanate, n- | 000111-36-4 |
| Butylamine, N-butylidene | 004853-56-9 |
| Carbodllmide, di-t-butyl- | 000691-24-7 |
| Chlorotoluene, p- | 000106-43-4 |
| Cinnamate, 2-ethylhexyl-4-methoxy- | 005466-77-3 |
| Cyanovaleric acid, 4- | 999900-00-3 |
| Cyclododecane | 000294-62-2 |
| Cyclohexadecane | 000295-65-8 |
| Cyclohexadiene-1-one, 2,6-(1,1-dimethylethyl)-4-methylene-2,5- | 002607-52-5 |
| Cyclohexanamine, 4,4'-methylene-bis- | 001761-71-3 |
| Cyclohexanamine, N-methyl- | 000100-60-7 |
| Cyclohexanamine, N-cyclohexyl- | 000101-83-7 |
| Cyclohexanamine, N,N-dimethyl- | 000098-94-2 |
| Cyclohexane, cis-1-methyl-4-isopropyl- | 006069-98-3 |
| Cyclohexane, 1-isopropyl-4-methyl- | 000099-82-1 |
| Cyclohexanemethanol, trans-alpha,alpha,4-trimethyl- | 005114-00-1 |
| Cyclohexanol | 000108-93-0 |
| Cyclohexanol, 3-methyl- | 000591-23-1 |
| Cyclohexanol, trimethyl- | 001321-60-4 |
| Cyclohexanol, 4-tert-butyl- | 000098-52-2 |
| Cyclohexanone, 2-(1-hydroxycyclohexyl)- | 028746-99-8 |
| Cyclohexen-1-one, 3-methyl-2- | 001193-18-6 |
| Cyclohexene, 4-cyano also (1-Cyano-3-cyclohexene) | 000100-45-8 |
| Cyclohexyl isocyanate | 003173-53-3 |
| Cyclohexylurea, dimethyl- | 031468-12-9 |
| Cyclopentanone | 000120-92-3 |
| Cyclopentylcyclopentanone, 2- | 004884-24-6 |
| Decadien-1-al, trans,trans-2,4- | 025152-84-5 D18 |
| Decadienal, 2,4- | 002363-88-4 |
| Decanamide, N,N-dimethyl- | 014433-76-2 |
| Decanedioic acid, bis(2,2,6,6-tetramethyl-4-piperidinyl)- | 052829-07-9 |
| Dehydroabietic acid | 001740-19-8 |
| Di-o-tolylguanidine, 1,3- | 000097-39-2 |
| Diazacyclotetradecane-2,9-dione, 1,8- | 056403-09-9 |
| Dibenzylamine | 000103-49-1 |
| Dibutyl cyanamide, N,N- | 002050-54-6 |
| 1,3-Dicyclohexylurea | 002387-23-7 |
| Diethylene glycol monomethacrylate homopolymer | 027598-43-2 |
| Diethylurea, 1,3- | 000623-76-7 |
| Dihydro-5-pentyl-2(3H)-furanone | 000104-61-0 |
| Dihydrobenzofuran, 2,3- | 000496-16-2 |
| Dihydrofuran, 4-methyl-2,3- | 034314-83-5 |
| Dihydromethoxymethyl oxopyridlnecarbonitrile | 000524-40-3 |
| Dihydromethyl benzimidazolone | 005400-75-9 |
| Dimethyl ditallow ammonium chloride | 068783-78-8 |
| Dimethyl thioacetamide | 000631-67-4 |
| Dimethyl-3,3'-thiobispropionate | 004131-74-2 |
| Dimethyl-p-benzoquinone, 2,5- | 000137-18-8 |
| Dimethylaminopyridine | 001122-58-3 |
| Dimethylbenzaldehyde, 2,4- | 015764-16-6 |
| Dimethylbenzaldehyde, 2,5 | 005779-94-2 |
| Dimethylbenzaldehyde, 3,4- | 005973-71-7 |
| Dimethylcyanamide | 001467-79-4 |
| Dimethyldiphenyl sulphone | 005097-12-1 |
| Dimethyldithiocarbamate, methyl | 003735-92-0 |
| Dimethyldodecanamide, N,N- | 003007-53-2 |
| Dimethylhexane-2,5-diol, 2,5- | 000110-03-2 |
| Dioxacyclododecane-7,12-dione, 1,6- | 000777-95-7 |
| Dioxathiocane, 1,3,6- | 002094-92-0 |
| Dioxolane-1,3, 4-ethyl | 029921-38-8 |
| Dithiolane-2-thione, 1,3- | 000822-38-8 |
| Docosane | 000629-97-0 D19 |
| Docosenamide (Erucamide) | 000112-84-5 |
| Docosenamide | 001120-16-7 |
| Dodecyl glycidyl ether | 0026461-18-9 |
| Ethan-1-one, 1-(methylphenyl)- | 026444-19-9 |
| Ethane, 1-(3-hydroxyphenyl)-2-phenyl- | 033675-75-1 |
| Ethanediamide, N-(2-ethoxyphenyl)-N-'-(2-ethylphenyl)- | 023949-66-8 |
| Ethanol, 2-[2-[2-[2-[(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethoxy]- | 049796-75-0 |
| Ethanol, 2-[2-[2-[(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethoxy]- | 058705-51-4 |
| Ethanol, 2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]- | 002315-61-9 |
| Ethanone, 1-[(4-hydroxy-3-methoxyphenyl)- | 000498-02-2 |
| Ethanone, 1-(4-(1-hydroxy-1-methylethyl)phenyl)- | 054549-72-3 |
| Ethyl hydroxyphthalide | 000485-26-7 |
| Ethylcyclopentanone | 004971-18-0 |
| Ethylene glycol dimethacrylate | 000097-90-5 |
| Fenchyl alcohol | 001632-73-1 |
| Fenchyl alcohol, alpha- | 000512-13-0 |
| Fenchyl alcohol, alpha- | 014575-74-7 |
| Fluorenone | 000486-25-9 |
| Formamide, N,N-diethyl- | 000617-84-5 |
| Formamide, N-methyl-N-phenyl- | 000093-61-8 |
| Formamide, N-cyclohexyl- | 000766-93-8 |
| Formamide, N-(1,1-dimethylethyl)- | 002425-74-3 |
| Formamide. N,N-di-n-butyl- | 000761-65-9 |
| Formamidine, N,N-dimethyl-N'-cyclohexyl- | 003459-75-4 |
| Formylcyclopentene, 1- | 006140-65-4 |
| Furan, tetrahydro-2,2,5,5-tetramethyl- | 015045-43-9 |
| Geraniol | 000106-24-1 |
| Guanidline, 1,2,3-triphenyl- | 000101-01-9 |
| Heptacosane | 000593-49-7 |
| Heptyl aldehyde, n- | 000111-71-7 |
| Hexacosane | 000630-01-3 |
| Hex-1-ene, 2-ethyl- | 001632-16-2 |
| Hex-5-en-1-ol | 000821-41-0 D20 |
| Hexadecanamide | 000629-54-9 |
| Hexadecanamide, N,N-dimethyl- | 003886-91-7 |
| Hexadecene-1 | 000629-73-2 |
| Hexamethylene oxide | 000592-90-5 |
| Hexamethylene dibenzamide | 005326-21-6 |
| Hexanal, 2-ethyl- | 000123-05-7 |
| Hexanal | 000066-25-1 |
| Hexane, 2,5-dimethyl- | 000592-13-2 |
| Hexane-2,5-dione | 000110-13-4 |
| Hexaoxacyclotriacontane, 1,6,11,16,21,26- | 064001-05-4 |
| Hexen-2-one, 5-methyl-5- | 003240-09-3 |
| Hydrocinnamic acid | 006386-38-5 |
| Hydroxydiphenylamine, 3- | 000101-18-8 |
| Hydroxypropyl methacrylate, 2- | 000923-26-2 |
| Icosane | 000112-95-8 |
| Imidazole, methylphenyl- | 000670-91-7 |
| Indan-1-one | 000083-33-0 |
| Indene, 2,3-dihydro- also (2,3-dihydro-1H-) | 000496-11-7 |
| Indene | 000095-13-6 |
| Isobutyramide | 000563-83-7 |
| Isocrotonic acid | 000503-64-0 |
| Isophorone diamine | 002855-13-2 |
| Isovanillin | 000621-59-0 |
| Laurolactam | 000947-04-6 |
| Methacrylate, lauryl- | 000142-90-5 |
| Methacrylic acid | 000079-41-4 |
| Methacrylic acid, 3-(trimethylsilyl)propyl ester | 002530-85-0 |
| Methane, di-t-butyl- | 001070-87-7 |
| Methoxybenzene | 000100-66-3 |
| Methyl anthranilate | 000134-20-3 |
| Methyl palmitate | 000112-39-0 |
| Methyl laurate | 000111-82-0 |
| Methyl stearate | 000112-68-1 |
| Methyl-4-isopropyl cyclohexane, trans-1- | 001678-82-6 D21 |
| Methyldiethyl carbamate | 004652-44-2 |
| Methylene bis(4-methyl-6-tertbutyl-phenol), 2,2' | 000119-47-1 |
| 2,2'-Methylenediphenol | 002467-02-9 |
| 4,4'-Methylenediphenol | 000620-92-8 |
| Methylenephenethyl alcohol, beta- | 006006-81-1 |
| Methylindene | 029036-25-7 |
| Methylpiperidine, 1- | 000626-67-5 |
| Methylthioacetonitrile | 035120-10-6 |
| Morpholine, methyl- | 000109-02-4 |
| Morpholinecarbaldehyde, 4- | 004394-85-8 |
| Morpholinepropanenitrile, 4- | 004542-47-6 |
| N-Butyl formamide | 000871-71-6 |
| N-Isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate | 000078-44-4 |
| Naphthalene, dimethyl- | 028804-88-8 |
| Naphthalene, ethyl | 027138-19-8 |
| Noanoic acid, n- | 000112-05-0 |
| Nonanal | 000124-19-6 |
| Nonanoic acid, 9-oxo- | 002553-17-5 |
| Norbornene, 5-ethylidene-2- | 016219-75-3 |
| Octadecadienoic acid, (Z,Z)-9, 12-, butyl ester | 002634-45-9 |
| Octadecenamide | 000301-02-0 |
| Octadecene, 1- | 000112-88-9 |
| Octadien-1-ol, 3,7-dimethyl-2,6- | 000624-15-7 |
| Octadien-2-ol, 2,6-dimethyl-5,7- | 005986-38-9 |
| Octadien-3-ol, 2,6-dimethyl-1,7- | 022460-59-9 |
| Octadien-3-ol, 3,7-dimethyl-1,6- | 000078-70-6 |
| Octadien-3-ol, 3,7-dimethyl-4,6- | 018479-54-4 |
| Octanal | 000124-13-0 |
| Octanoate, methyl- | 000111-11-5 |
| Octaphenyl pentaethylene glycol ether, tert- | 038621-31-7 |
| Octen-3-ol, 1- | 003391-86-4 |
| Octylphenoxypentaethoxyethanol, tert- | 037809-81-7 |
| Oleate, n-butyl- | 000142-77-8 D22 |
| Oxabicyclo (4.1.0) heptane-3-carboxylic acid, 7- | 002386-87-0 |
| Oxamide, di-tert-butyl- | 037486-48-9 |
| Oxaspirodecadienedione, di-(t-butyl) | 082304-66-3 |
| Oxirane, [(dodecytoxy)methyl]- | 002461-18-9 |
| Oxybis(propanenitrile) | 001656-48-0 |
| Palmitate, isopropyl- | 000142-91-6 |
| Pentacosane | 000629-99-2 |
| Pentane, 1-amino | 000110-58-7 |
| Pentanediol, 2,2,4-trimethyl-1,3- | 000144-19-4 |
| Pentanenitrile | 000110-59-8 |
| Pentaoxacyclopentacosane, 1,6,11,16,21- | 056890-57-4 |
| Pentenal, trans-2- | 001576-87-0 |
| Peroxide, tert-butyl- | 000110-05-4 |
| Phenanthrene | 000085-01-8 |
| Phenol, 4-ethoxy- | 000622-62-8 |
| Phenol, 0-(1-phenylethyl)- | 004237-44-9 |
| Phenol, (phenylethyl)- | 051937-33-8 |
| Phenol, o-(alpha, alpha-dimethylbenzyl)- | 018168-40-6 |
| Phenol, p-(alpha, alpha-dimethylbenzyl)- | 000599-64-4 |
| Phenol, p-phenylethyl- | 006335-83-7 |
| Phenol, 4-(2-propentyl)- | 000501-92-8 |
| Phenol, 3,5-dibenzyl-2,4,6-trimenthyl- | 999900-00-1 |
| Phenol, 2,6-di-t-butyl-4-methoxy- | 000489-01-0 |
| Phenol, 4-(1-phenylethyl)- | 001988-89-2 |
| Phenol, 2-allyl- | 001745-81-9 |
| Phenyl isothiocyanate | 000103-72-0 |
| Phenyl-1-buten-4-ol, 4- | 000936-58-3 |
| Phenylbutane, 2- | 000135-98-8 |
| Phenylene) bis-ethanone, 1,1'-(1,4- | 001009-61-6 |
| Phenylene) bis-ethanone, 1,1'-(1,3- | 006781-42-6 |
| 2,2'-p-Phenylenedioxydiethanol | 000104-38-1 |
| Phenylethanol, 2- | 000060-12-8 |
| (Phenylimino) cyclohexadiene | 002406-04-4 |
| Phosphate, diphenyl-2-ethylhexyl- | 001241-94-7 D23 |
| Phosphonic acid, (nitrilotris(methylene)) tri-, pentasodium | 002235-43-0 |
| Phthalide [also 1(3H)-isobenzofuranone] | 000087-41-2 |
| Pinanol | 000473-54-1 |
| Pinanol (or cis-2-pinanol) | 004948-28-1 |
| Pinacampheol (also Pinocamphone) | 000547-60-4 |
| Piperidine, 1-formyl | 002591-86-8 |
| Piperidine, 2-propyl- | 000458-88-8 |
| Piperidinol, 1,2,2,6,6-pentamethyl-4- | 002403-89-6 |
| Poly(oxy-1,2-ethanediyl), a-isotridecyl-w-hydroxy-, phosphate | 073038-25-2 |
| Propanal, 2,2-dimethyl-3-hydroxy- | 000597-31-9 |
| Propanaminium chloride, N,N,N-trimethyl-3-((1-oxo-2-propenyl)amino)-1- | 045021-77-0 |
| Propane, 1,1-dimethoxy-2-methyl | 041632-89-7 |
| Propanediol, 2-ethyl-2-butyl-1,3- | 000115-84-4 |
| Propanenitrile, 3,3'-oxybis- | 001656-48-0 |
| Propanenitrile, 3,3'-thiobis- | 000111-97-7 |
| Propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanedlyl | 074381-40-1 |
| Propanoic acid, 3-ethoxy-, ethyl ester | 000763-69-9 |
| Propanoic acid, 2,2-dimethyl- | 000075-98-9 |
| Propanoic acid, 2-methyl-, 3-hydroxy-2,4,4-trimethylpentyl ester | 000077-68-9 |
| Propanoic acid, 2-methyl, 3-hydroxy-2,4,4-trimethylpentyl ester | 074367-34-3 |
| Propanoic acid, 2-methyl, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)propyl | 074367-33-2 |
| Propanol, 1-amino-2- | 000078-96-6 |
| Propanol, phenyl-1- | 001335-12-2 |
| Propanol, 1-propoxy-2- | 001569-01-3 |
| Propanone, 1-phenyl-1- | 000093-55-0 |
| Propenoic acid, 2-methyl-2-, polymer with octadecyl-2-methyl-2- | 027401-06-05 |
| Propenone, (dihydroxy methoxyphenyl) phenyl- | 018956-15-5 |
| Pyrene | 000129-00-0 |
| Pyridine, 2,4-dimethyl- | 000108-47-4 |
| Pyridine, 2,4,6-trimethyl- | 000108-75-8 |
| Pyridine, 1,2,3,4-tetrahydro-1,2,2,6-tetramethyl- | 063867-76-5 |
| Pyridine, 1,2,3,6-tetrahydro-1,2,3,4-tetramethyl- | 090949-18-1 |
| Pyridine, 1,2,3,6-tetrahydro-1,2,4,5-tetramethyl- | 090949-19-2 D24 |
| Pyridine, 1,2,3,6-tetrahydro-1,2,4,6-tetramethyl-, cis- | 023513-16-8 |
| Pyridine, 1,2,3,6-tetrahydro-1,3,3,6-tetramethyl- | 122913-54-6 |
| Pyridine, 1,2,3,6-tetrahydro-1,4,5,6-tetramethyl- | 090949-20-5 |
| Pyridine, 1,2,3,6-tetrahydro-2,2,2,6-tetramethyl- | 001124-69-2 |
| Pyridine, 1,2,5,6-tetrahydro-2,2,5,5-tetramethyl- | 155904-89-5 |
| Pyridine, 2,3,4,5-tetrahydro-2,2,4,6-tetramethyl- | 200561-41-7 |
| Pyrrolidine | 000123-75-1 |
| Sodium p-sulfophenyl methallyl ether | 001208-67-9 |
| Squalene | 007683-64-9 |
| Styrene, alpha-methyl- | 000098-83-9 |
| Styrene, methyl- (mixed isomers) | 025013-15-4 |
| Sulfonylbis(4-methyl)-benzene, 1,' | 000599-66-6 |
| Terephthalic acid, monomethyl ester | 001679-64-7 |
| Terpineol, alpha- | 000098-55-5 |
| tert-Butylamine | 000075-64-9 |
| Tetracosane | 000646-31-1 |
| Tetradecanamide | 000638-58-4 |
| Tetradecane | 001120-36-1 |
| Tetraethyleneglycol di-(2-ethylhexoate) | 018268-70-7 |
| Tetraethyleneglycol dimethacrylate | 000109-17-1 |
| Tetrahydrofuran, diphenyl- | 050637-09-7 |
| Tetrahydropyridine, 2,3,4,5- | 000505-18-0 |
| Tetramethyl urea | 000632-22-4 |
| Tetramethyldec-5-yne-4,7-diol, 2,4,7,9- | 000126-86-3 |
| Tetramethyldecynediol | 001333-17-1 |
| 2,6,10, 14- Tetramethylhexadecane | 000638-36-8 |
| Tetramethylpyrazine, 2,3,5,6- | 001124-11-4 |
| Tetramethylsuccinonitrile | 003333-52-6 |
| Tetraoxacycloeicosane, 1,6,11, 16- | 017043-02-6 |
| 4,4'-Thiobis-(6-t-butyl-o-cresol) | 000096-66-2 |
| Triallyl cyanurate | 000101-37-1 |
| Tributoxyethyl phosphate | 000078-51-3 |
| Tributylphosphine oxide | 000814-29-9 |
| Trichlorotrifluoroethane | 026523-64-8 D25 |
| Tricosane also (n-tricosane) | 000638-67-5 |
| Triethylamine | 000121-44-8 |
| Triethyleneglycol dimethacrylate | 000109-16-0 |
| Triethylsilanol | 000597-52-4 |
| Trimethylcyclohexanone | 050874-76-5 |
| Trimethylolpropane trimethacrylate | 003290-92-4 |
| Trioxepane | 005981-06-6 |
| Triphenylphosphate | 000115-86-6 |
| Triphenylphosphine oxide | 000791-28-6 |
| Urea, N,N-bis-(1, 1-dimethylethyl)- | 005336-24-3 |
| Valeronitrile, 2,4-dimethyl- | 034372-09-3 |
| 1) For the chemicals listed in this table, the evaluation criteria are 0.003 mg/L under static conditions, and 0.003 mg/L under flowing conditions. | |
The drinking water criteria in this annex have not undergone external peer review.
The drinking water criteria in this annex are intended to be used as guidance in the determination of evaluation criteria for those compounds that do not have normative evaluation criteria established. Some of these values, as noted in the tables, are currently under external peer-review for inclusion as normative criteria. The values in these tables include criteria that have been developed according to the requirements of annex A, but have not been externally peer-reviewed. The tables also include non-regulatory USEPA values that have been reviewed but failed to satisfy annex A toxicity data requirements. Compounds that have been detected only at concentrations below the threshold of evaluation (see annex A, A.2.7.1) to which the threshold of evaluation protocol has been applied are also listed here.
The drinking water criteria in this annex have not been evaluated for taste and odor considerations at the concentration limits indicated.
In the event that one of the chemicals listed in this annex is detected at concentrations exceeding the guidance evaluation criteria values, a complete toxicity data review should be conducted. The review should be performed according to annex A requirements prior to using the informational evaluation criteria values to determine product compliance to this Standard.
The substances listed in tables E1 and E2 are not intended to encompass all of the potential analytes of interest that need to be considered when evaluating products. The user is cautioned that each product may have formulation dependent analytes of interest for which concentration limits have not been determined. In these cases, the user is required to develop acceptable concentration limits based on the requirements of annex A of NSF/ANSI 61 in order to determine full compliance with the Standard.
These tables are specific to NSF/ANSI 61. While the tables may be used for evaluation of impurities in drinking water treatment chemicals, the substances listed in these tables may have not been evaluated for use as direct additive drinking water treatment chemicals under NSF/ANSI 60. Use as direct additive drinking water treatment chemicals may require the consideration of different exposure parameters than those used for NSF/ANSI 61 evaluation.
Table F1 contains drinking water criteria for unregulated contaminants that have been identified as extractants from products covered by this Standard. For criteria set by NSF International, the TAC and SPAC criteria have been determined in accordance with annex A of NSF/ANSI 61. External peer review is in progress on these evaluation criteria, as noted in the table. As external peer review is completed, those criteria will be submitted for inclusion as normative evaluation criteria in this Standard.
Table E1 also contains drinking water criteria for unregulated contaminants that have had guidance values set by USEPA. These criteria are derived from the USEPA IRIS online database or from USEPA Health Advisories, but the TAC and SPAC values are limited by the toxicity data requirements for Level I, II, III, or IV in NSF/ANSI 61 (see annex A, A.2.7). The concentrations are generally limited to Level II (50 ppb), because reproductive, developmental, and/or chronic studies are not available. In the absence of sufficient information to determine
1) This annex was annex F in ANSI/NSF 61 – 2000.
2) The information contained in this Annex is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI's requirements for an ANS. As such this Annex may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard.
E1a data-derived relative source contribution factor, a default 20% drinking water contribution is assumed.
Some of the SPAC values do not represent 10% of the corresponding TAC values: either a data deficiency precluded setting of the TAC at a higher value, or a data-derived multiple source factor other than the 10% default value was applied.
Table E2 contains chemicals that have been evaluated using the threshold of evaluation (see annex A, A.2.7.1), but that may have sufficient toxicity data available that would enable chemical specific risk assessments to be performed if needed. To date, these chemicals have not been detected at concentrations exceeding the threshold of evaluation criteria. In the event that these chemicals are detected at concentrations exceeding the threshold of evaluation criteria, a toxicity data review should be conducted according to annex A prior to using the threshold of evaluation to determine product compliance to this Standard.
E2| Substance | CAS # | Total Allowable Concentration (TAC) mg/L |
Single Product Allowable Concentra-tion (SPAC) mg/L |
Source of supporting documentation1) |
|---|---|---|---|---|
| Inorganics | ||||
| Bismuth | 7440-69-9 | 0.05 | 0.01 (non-section 9)2) 0.05 (section 9)2) |
NSF action levels3) Issue date: 08/02/95 |
| Lithium | 7439-93-2 | 1.0 | 0.3 | NSF action levels3) Issue date: 09/27/99 |
| Nickel | 7440-02-0 | 0.1 | 0.02 | NSF action levels3) Issue date: 07/22/96 |
| Strontium | 7440-24-6 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 06/23/92 |
| Vanadium | 7440-62-2 | 0.03 | 0.003 | NSF action levels3) Issue date: 02/11/00 |
| Zinc | 7440-66-6 | 3 | 0.3 | EPA Longer-term Drinking Water Health Advisory for a child Issue date: 1992 |
| Organics | ||||
| Acetaldehyde | 75-07-0 | 0.01 | 0.01 | NSF action levels3) Issue date: 04/24/96 |
| Acetone | 67-64-1 | 1 | 0.6 | NSF action levels3) Issue date: 06/23/93 |
| 11-Aminoundecanoic acid | 2432-99-7 | 0.05 | 0.05 | NSF action levels3) In external peer review as of 04/15/99 |
| Benzyl alcohol | 100-51-6 | 0.05 | 0.05 | NSF action levels3) Issue date: 11/03/97 |
| Bisphenol A | 80-05-7 | 0.2 | 0.02 | NSF action levels3) Issue date: 02/06/97 E3 |
| Bisphenol A Diglycideryl ether | 1675-54-3 | 0.004 | 0.0004 | NSF action levels, based on epichlorohydrin3) Issue date: 2/09/99 |
| Bisphenol A Diglycideryl ether | None Available | 0.004 | 0.0004 | NSF action levels, based on epichlorohydrin3) Issue date: 2/09/99 |
| 1,3-Butadiene | 106-99-0 | 0.0002 | 0.00002 | NSF action levels3) Issue date: 1/12/96 |
| Butyl acrylate | 141-32-2 | 0.01 | 0.01 | NSF action levels3) Issue date: 12/13/95 |
| tert - Butyl alcohol | 75-65-0 | 0.05 | 0.05 | NSF action levels3) In external peer review as of 04/15/99 |
| Butylbenzyl phthalate | 85-68-7 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 06/15/89 |
| tert - Butyl hydroquinone | 1948-33-0 | 0.01 | 0.01 | NSF action levels3) Issue date: 11/03/95 |
| Butyltin compounds (mono- and di- only) | N/A | 0.02 (Total) | 0.004 (Total) | NSF action levels3) Issue date: 12/19/91 |
| Carbon disulfide | 75-15-0 | 0.05 | 0.05 | NSF action levels3) Issue date: 11/15/93 |
| Cyclohexanone | 108-94-1 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental data has been applied. Verification date: 09/02/86 E4 |
| Dibutylamine | 111-92-2 | 0.01 | 0.01 | NSF action levels3) Issue date: 08/19/95 |
| Di-n-butyl phthalate | 84-74-2 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 01/22/86 |
| Dichloropropanol includes: 2,3-Dichloro-1-propanol 1,3-Dichloro-2-propanol |
26545-73-3 616-23-9 96-23-1 |
0.03 (Total) | 0.009 (Total) | NSF action levels3) Issue date: 5/97 |
| Diethylene triamine | 111-40-0 | 0.01 | 0.01 | NSF action levels3) Issue date: 11/14/97 |
| Diethyl phthalate | 84-66-2 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 07/16/87 |
| Diisononyl phthalate | 28553-12-0 | 0.05 | 0.05 | NSF action levels3) Issue date: 04/06/98 |
| 2,4-Dimethylphenol | 105-67-9 | 0.05 | 0.01 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 02/21/90 E5 |
| Dimethylterephthalate | 120-61-6 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental data has been applied. Verification date: 10/09/85 |
| Diphenylamine | 122-39-4 | 0.05 | 0.02 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of developmental data has been applied. Verification date: 07/22/86 |
| 1,4-Dithiane | 505-29-3 | 0.05 | 0.007 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 06/24/92 |
| Ethanolamine | 141-43-5 | 0.01 | 0.01 | NSF action levels3) Issue date: 09/17/96 |
| Ethyl acrylate | 140-88-5 | 0.01 | 0.001 | NSF action levels3) Issue date: 01/28/92 |
| Ethylenediamine | 107-15-3 | 2 | 0.2 | NSF action levels3) Issue date: 11/27/99 |
| Ethyl-4-ethoxybenzoate | 23676-09-7 | 0.05 | 0.05 | NSF action levels3) Issue date: 11/17/99 |
| 2-Ethyl-1-hexanol | 104-76-7 | 0.05 | 0.05 | NSF action levels3) Issue date: 03/29/95 |
| Hexamethylsiloxane | 107-46-0 | 0.01 | 0.01 | NSF action levels3) Issue date: 08/15/95 E6 |
| Hydroquinone | 123-31-9 | 0.006 | 0.0006 | NSF action levels3) Issue date: 04/16/92 |
| Isophthalic acid | 121-91-5 | 0.01 | 0.01 | NSF action levels3) Issue date: 12/18/95 |
| Maleic acid | 110-16-7 | 0.05 | 0.05 | NSF action levels3) Issue date: 05/26/95 |
| 2- Mercaptobenzothiazole | 149-30-4 | 0.04 | 0.004 | NSF action levels3) Issue date: 11/05/93 |
| Methanol | 67-56-1 | 4 | 2 | NSF action levels3) In external peer review as of 08/06/98 |
| 4,4'-Methylenedianiline | 101-77-9 | 0.001 | 0.0001 | NSF action levels3) Issue date: 06/90 |
| Methyl ethyl ketone (MEK) | 78-93-3 | 1 | 0.6 | NSF action levels3) Issue date: 12/16/91 |
| Methyl methacrylate | 80-62-6 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental data has been applied. Agency Consensus Date: 11/25/97 |
| 1-Methylnaphthalene | 90-12-0 | 0.05 | 0.05 | NSF action levels3) Issue date 09/16/96 |
| 2-Methylnaphthalene | 91-57-6 | 0.01 | 0.01 | NSF action levels3) Issue date: 09/13/96 |
| N-Methyl-2-pyrrolidone | 872-50-4 | 1 | 0.1 | NSF action levels3) Issue date: 06/17/93 |
| Methyltin compounds (mono- and di- only) | N/A | 0.03(Total) | 0.006 (Total) | NSF action levels3) Issue date: 12/19/91 E7 |
| Morpholine | 110-91-8 | 0.01 | 0.01 | NSF action levels3) Issue date: 10/17/95 |
| Naphthalene | 91-20-3 | 0.05 | 0.01 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic oral data, plus quantitative data on hemolytic anemia has been applied. Agency Consensus Date: 07/01/98 |
| Nitroguanidine | 556-88-7 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 05/17/89 |
| Pentachloronitrobenzene | 82-68-8 | 0.02 | 0.002 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for the lack of reproductive/ developmental and chronic data has been applied. Verification date: 04/15/87 |
| Phenol | 108-95-2 | 0.01 | 0.01 | NSF action levels3) Issue date: 04/10/95 |
| 2-Phenyl-2-propanol | 617-94-7 | 0.05 | 0.01 | NSF action levels3) In external peer review as of 04/15/99 E8 |
| Phthalic anhydride | 85-44-9 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 02/24/88 |
| Propylene glycol monomethyl ether | 107-98-2 | 0.05 | 0.05 | NSF action levels3 Issue date: 2/04/94 |
| Sodium diethyldithiocarbamate | 148-18-5 | 0.05 | 0.02 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 10/09/85 |
| Terephthalic acid | 100-21-0 | 0.01 | 0.01 | NSF action levels3 Issue date: 01/10/97 |
| Tetrahydrofuran | 109-99-9 | 1 | 0.37 | NSF action levels3 Issue date: 01/26/96 |
| Tetramethylthiourea | 2782-91-4 | 0.002 | 0.0002 | NSF action levels3 Issue date: undated |
| Toluene dilsocyanate 2,4- (80%) and 2,6-(20%) (for specified mixture only) | 26471-62-5 | 0.008 | 0.0008 | NSF action levels3 Issue date: –6/99 |
| Trichlorofluoromethane | 75-69-4 | 0.05 | 0.05 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contributions for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 05/31/85 E9 |
| Triethanolamine | 102-71-6 | 0.05 | 0.05 | NSF action levels3 In external peer review as of 04/13/00 |
| Triethyl phosphate | 78-40-0 | 0.01 | 0.01 | NSF action levels3 Issue date: 11/99 |
| Trimethylamine | 75-50-3 | 0.01 | 0.001 | NSF action levels3 Issue date: 11/11/96 |
| 1,3,5-Trinitrobenzene | 99-35-4 | 0.05 | 0.02 | Derived from the oral RfD on the EPA IRIS database with a default 20% relative source contribution for drinking water. Concentration limitation for lack of reproductive/developmental and chronic data has been applied. Verification date: 08/27/97 |
| Vinyl acetate | 108-05-4 | 0.02 | 0.002 | NSF action levels3 Issue date: 05/03/91 |
| 1) Criteria are derived from the oral RfD on the EPA IRIS database as follows: Oral RfD (mg/kg-day) × (70 kg / 2L/day) × relative source contribution factor = TAC (mg/L) Where: 70 kg = assumed adult body weight 2L/day = assumed adult water consumption relative source contribution factor = percentage of daily exposure to the substance represented by drinking water (default value is 20%) 2) For Section 9 products, a 100% multiple source factor was applied during the SPAC calculation, since no other sources of bismuth were expected within the one liter draw specified for Section 9. For non-Section 9 products, a 20% multiple source factor was applied. 3) NSF action levels have been derived according to the requirements of ANSI/NSF 61 - 1999a, annex A. External peer review is in progress on some of these substances, as noted. |
||||
| Substance | CAS # |
|---|---|
| Inorganics | |
| Cobalt | 7440-48-4 |
| Sodium thiocyanate | 540-72-7 |
| Titanium | 7440-32-6 |
| Organics | |
| Benzene, 1-chioro-4-(trifluoromethyl) | 98-56-6 |
| Benzamide | 55-21-0 |
| Benzotriazole, 1,2,3- | 95-14-7 |
| Benzyl acetate | 140-11-4 |
| Benzyl alcohol, 3,5-di-tert-butyl-4-hydroxy- | 88-26-6 |
| Butyl acetate | 123-86-4 |
| Cyanoguanidine | 461-58-5 |
| Dichlorodifluoromethane | 75-71-8 |
| Dichlorophenol, 2,4- | 120-83-2 |
| Diethylaminoethanol | 100-37-8 |
| Dimethylacetamide, N,N- | 127-19-5 |
| Dimethyl adipate | 627-93-0 |
| Dimethylaminopropanenitrile | 1738-25-6 |
| Dimethylformamide, N,N- | 68-12-2 |
| Dimethyl phthalate | 131-11-3 |
| Diphenyl guanidine, 1,3- (or N,N-) | 102-06-7 |
| Diphenyl-p-phenylenediamine, N,N'- | 74-31-7 |
| Ethanol, 2-diethylamino- | 100-37-8 |
| Ethanol, 2-phenoxy- | 122-99-6 |
| Ethanol, 1-phenyl- | 98-85-1 |
| Fluoranthene | 206-44-0 |
| Furanmethanol, 2- | 98-00-0 |
| Hexamethylenediamine | 124-09-4 E11 |
| Hexamethylenetetramine | 100-97-0 |
| Hexanoic acid, 2-ethyl | 149-57-5 |
| Isobutyl isobutyrate | 97-85-8 |
| (Isopropylamino)diphenylamine, 4- | 101-72-4 |
| Isopropyltoluene | 99-87-6 |
| Methyl acrylate | 96-33-3 |
| Methyldiethanolamine, N- | 105-59-9 |
| Methylene diphenyl diisocyanate | 101-68-8 |
| Methylene bis(N-iso-butylbenzenamine) | 88990-59-4 |
| Phenylene diamine, N-(1,3-dimethylbutyl)-N3-phenyl-p- | 793-24-8 |
| Phenylenediamine, N-phenyl-p- | 101-54-2 |
| Phthalic acid, o- | 88-99-3 |
| Piperidine | 110-89-4 |
| Sebacate, bis(2-ethylhexyl)- | 122-62-3 |
| Silane, gamma-aminopropyl triethoxy- | 919-30-2 |
| Tacrine | 321-64-2 |
| Tetramethylene sulfone | 126-33-0 |
| Tetramethyl piperidinone | 826-36-8 |
| Triallyl isocyanurate | 1025-15-6 |
| Triethylene diamine | 280-57-9 |
| Tris(2-ethylhexyl) phosphate | 78-42-2 |
| Vanillin, o- | 148-53-8 |
| 1) For the chemicals in this table, the evaluation criteria are 0.003 mg/L under static conditions and 0.0003 mg/L under flowing conditions. The chemicals that appear in this table have been detected only at concentrations not exceeding these threshold of evaluation concentrations as established in this Standard (see annex A, section A.2.7.1), and have not been evaluated for specific TAC and SPAC values. If any of these chemicals are detected at concentrations exceeding the threshold of evaluation, toxicity data shall be reviewed to determine whether specific TAC and SPAC values can be established, prior to using threshold of evaluation to determine compliance with the Standard. | |

THE MOST TRUSTED CERTIFICATION IN THE DRINKING WATER INDUSTRY
E13

The information contained in this section is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI's requirements for an ANS. As such, this section may contain material that has not been subjected to public review of a consensus process, In addition, it does not contain requirements necessary for conformance to the Standard.
E16
Standards and Criteria4
The following standards and criteria established and adopted by NSF as minimum voluntary consensus standards are used internationally:
| 2 | Food equipment |
| 3 | Commercial warewashing equipment |
| 4 | Commercial cooking, rethermalization, and powered hot food holding and transport equipment |
| 5 | Water heaters, hot water supply boilers, and heat recovery equipment |
| 6 | Dispensing freezers |
| 7 | Commercial refrigerators and freezers |
| 8 | Commercial powered food preparation equipment |
| 12 | Automatic ice making equipment |
| 13 | Refuse processors and processing systems |
| 14 | Plastics piping system components and related materials |
| 18 | Manual food and beverage dispensing equipment |
| 20 | Commercial bulk milk dispensing equipment |
| 21 | Thermoplastic refuse containers |
| 24 | Plumbing system components for manufactured homes and recreational vehicles |
| 25 | Vending machines for food and beverages |
| 29 | Detergent and chemical feeders for commercial spray-type dishwashing machines |
| 35 | High pressure decorative laminates (HPDL) for surfacing food service equipment |
| 36 | Dinnerware |
| 37 | Air curtains for entranceways in food and food service establishments |
| 40 | Residential wastewater treatment systems |
| 41 | Non-liquid saturated treatment systems |
| 42 | Drinking water treatment units— Aesthetic effects |
| 44 | Residential cation exchange water softeners |
| 46 | Evaluation of components and devices used in wastewater treatment systems |
| 49 | Class II (laminar flow) biohazard cabinetry |
| 50 | Circulation system components and related materials for swimming pools, spas/hot tubs |
| 51 | Food equipment materials |
| 52 | Supplemental flooring |
| 53 | Drinking water treatment units—Health effects |
| 55 | Ultraviolet microbiological water treatment systems |
| 58 | Reverse osmosis drinking water treatment systems |
| 59 | Mobile food carts |
| 60 | Drinking water treatment chemicals — Health effects |
| 61 | Drinking water system components — Health effects |
| 62 | Drinking water distillation systems |
| 75 | Non-potentially hazardous foods |
| 116 | Non-food compounds used in food processing facilities – Food grade lubricants (draft standard for trial use) |
| 173 | Dietary supplements (draft standard for trial use) |
| 184 | Residential dishwashers |
| 14159 | Safety of machinery—Hygiene requirements for the design of machinery |
| 14159-1 | Hygiene requirements for the design of meat and poultry processing equipment C-2 Special equipment and/or devices |
4 The information contained in this Standards and Criteria page is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI's requirements for an ANS. As such, this Standards and Criteria page may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard.
E18
THE HOPE OF MANKIND rests in the ability of man to define and seek out the environment which will permit him to live with fellow creatures of the earth, in health, in peace, and in mutual respect.
Printed 1/15/02
ANSF/ANSI 61-2001
Addendum 1.0 - 2001
Drinking water system components—Health effects
NSF International Standard/American National Standard
Developed by a consortium of:
With support from:

NSF International, an independent, not-for-profit organization, is dedicated to public health, safety and protection of the environment by developing standards, by providing education and by providing superior third-party conformity assessment services while representing the interest of all stakeholders.
This Addendum is intended to replace the specific sections of NSFIANSI 61 — 2001 referenced. Contact NSF to confirm this revision is current.
Users of this Addendum may request clarifications and interpretations, or propose revisions by contacting:
Chair, Joint Committee on Drinking Water Additives
c/o NSF International
789 North Dixboro Road, P.O. Box 130140
Ann Arbor, Michigan 48113-0140 USA
Phone: (734) 769-8010 Telex: 753215 NSF INTL
FAX: (734) 769-0109 E-mail: info@nsf.org
Web: http://www.nsf.org
NSF/ANSI 61 – 2001
Addendum 1.0 – 2001
NSF International Standard/American National Standard for Drinking Water Additives —
Drinking water system components — Health effects
Standard Developer
NSF International
Adopted September 28, 2001
NSF International
Designated as an ANSI Addendum
September 28, 2001
American National Standards Institute
Prepared by
The NSF Joint Committee on Drinking Water Additives
The NSF Council of Public Health Consultants
Adopted by
NSF International
June 1988
Revised October 1988
Revised May 1990
Revised May 1991
Revised May 1992
Revised September 1994
Revised January 1995
Revised July 1996
Revised September 1996
Revised November 1996
Revised January 1997
Revised March 1997
Revised July 1997
Revised November 1998
Revised January 1999
Revised November 1999
Revised September 2000
Revised November 2000
Revised February 2001
Addendum 1.0, September 2001
Published by
NSF International
PO Box 130140, Ann Arbor, Michigan 48113-0140, USA
For ordering copies or for making inquiries with regard to this Addendum, please reference the designation “NSF/ANSI 61 — 2001, Addendum 1.0 — 2001.”
Copyright 2001 NSF International
Previous editions © 2000, 1999, 1998, 1997, 1996, 1995, 1994, 1992, 1991, 1990, 1988
Unless otherwise specified, no part of this publication may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and microfilm, without permission in writing from NSF International.
Printed in the United States of America.
iiDisclaimers1
NSF, in performing its functions in accordance with its objectives, does not assume or undertake to discharge any responsibility of the manufacturer or any other party. The opinions and findings of NSF represent its professional judgment. NSF shall not be responsible to anyone for the use of or reliance upon this Addendum by anyone. NSF shall not incur any obligation or liability for damages, including consequential damages, arising out of or in connection with the use, interpretation of, or reliance upon this Addendum.
NSF Standards provide basic criteria to promote sanitation and protection of the public health. Provisions for mechanical and electrical safety have not been included in this Addendum because governmental agencies or other national standards-setting organizations provide safety requirements.
Participation in NSF Standards development activities by regulatory agency representatives (federal, local, state) shall not constitute their agency’s endorsement of NSF or any of its Standards or Addendums.
Preference is given to the use of performance criteria measurable by examination or testing in NSF Standards development when such performance criteria may reasonably be used in lieu of design, materials, or construction criteria.
The illustrations, if provided, are intended to assist in understanding their adjacent standard requirements. However, the illustrations may not include all requirements for a specific product or unit, nor do they show the only method of fabricating such arrangements. Such partial drawings shall not be used to justify improper or incomplete design and construction.
Unless otherwise referenced, the annexes are not considered an integral part of NSF Standards and Addendums. The annexes are provided as general guidelines to the manufacturer, regulatory agency, user, or certifying organization.
1 The information contained in this Disclaimer is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI’s requirements for an ANS. As such, this Disclaimer may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard.
iii iv| A. 10.2 Physical and chemical properties | A1 |
| Table D4 – Threshold of evaluation chemicals | D1 |
| Table E2 – Threshold of evaluation chemicals having datasets from which specific TAC/SPAC values, or CBEL values, could possibly be set using Annex A | E1 |
© 2001 NSF NSF/ANSI 61 — 2001
Addendum 1.0 - 2001
Addendum to NSF/ANSI Standard for Drinking Water Treatment Units —
Drinking water system components — Health effects
A. 10.2 Physical and chemical properties2
The assessment shall define the following parameters for the substance, as applicable:
– chemical formula, structure, CAS number, and molecular weight;
– physical state and appearance;
– melting point or boiling point;
– vapor pressure;
– solubility in water;
– density;
– organoleptic properties (taste and odor thresholds);
– dissociation constant (pKa); and
– partition coefficients (octanol/water, air/water).
2 Annex A is a normative annex and followed ANSI procedures for inclusion into an ANSI standard.
A1 A2| Substance | CAS # |
|---|---|
| Inorganics | |
| yttrium | 007440-65-5 |
| Organics | |
| acenaphthylene | 000208-96-8 |
| acetophenone, p-isopropyl- | 000645-13-6 |
| acetophenone, 2'-methyl- | 000577-16-2 |
| acetophenone, 4-methyl | 000122-00-9 |
| acetophenone, alpha-hydroxy- | 000582-24-1 |
| acetophenone, 3'-methyl- | 000585-74-0 |
| acetophenone, 4'-isopropenyl | 005359-04-6 |
| acetophenone, 4'-hydroxy- | 000099-93-4 |
| alcohols, C12-C15, ethoxylated propoxylated | 068551-13-3 |
| allyl phenol ether | 001746-13-0 |
| aminoundecanoic acid, 12- | 000693-57-2 |
| benzaldehyde, 3,5-di-tert-butyl-4-hydroxy- | 001620-98-0 |
| benzaldehyde, 4-hydroxy-3-methoxy (Vanillin) | 000121-33-5 |
| benzaldehyde, 3,5-dimethoxy-4-hydroxy- | 000134-96-3 |
| benzaldehyde, 2-hydroxy- | 000090-02-8 |
| benzaldehyde, 2-hydroxy-4-methoxy | 000673-22-3 |
| benzaldehyde, hydroxymethoxy- | 106799-60-4 |
| benzaldehyde, tert-butylmethyl- | 066949-23-3 |
| benzene, 2-ethoxyethenyl- | 017655-74-2 |
| benzene, (2-methoxy-1-methylethyl)- | 065738-46-7 |
| benzene, divinyl- | 001321-74-0 |
| benzene, (1-methoxy-1-methylethyl)- | 000935-67-1 |
| benzene, 1, 1-oxybis- | 000101-84-8 |
| benzene, 1, 3-Dimethyl-5-isopropyl- | 004706-90-5 |
| benzene, 4, 6-diisopropyl-1,3-dimethyl- | 005186-68-5 |
| benzeneacetaldehyde | 000122-78-1 |
| benzenediamine, ar,ar-diethyl-ar-methyl | 068479-98-1 |
| benzenedimethanol, a,a,a',a'-tetramethyl-1,4- | 002948-46-1 |
| benzenedimethanol, a,a,a',a'-tetramethyl-1,3- | 001999-85-5 |
| benzenemethanamine, N-(phenylmethylene)- | 000780-25-6 |
| benzenemethanol, 4-(1-methylethyl)- | 000536-60-7 |
| benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy- | 020170-32-5 |
| benzenesulfonamide, 4-methyl- | 000070-55-3 |
| benzenesulfonyl isocyanate, 4-methyl | 004083-64-1 |
| benzenetricarboxylic acid, 1,2,4- | 000528-44-9 |
| benzimidazolone, 3-methyl-2- | 001849-01-0 |
| benzofuran, methyl- | 025586-38-3 |
| benzoic acid, m-methyl- | 000099-04-7 |
| benzoic acid, 4-tert-butyl- | 000098-73-7 |
| benzoic acid, ethyl ester | 000093-89-0 |
| benzoic acid, methyl ester | 000093-58-3 |
| benzonitrile | 000100-47-0 |
| benzoquinone, 2,6-di-t-butyl- | 000719-22-2 |
| benzoquinone, 2,5-di-tert-pentyl-p- | 004584-63-8 |
| benzothiazole | 000095-16-9 |
| benzothiazole, ethylamino- | 028291-69-2 |
| benzothiazole, 2-(methylmercapto)- | 000615-22-5 D1 |
| benzothiazole, 2-methyl- | 000120-75-2 |
| benzothiazole, 2-(morpholinothio)- | 000102-77-2 |
| benzothiazole-2-thione, N-methyl- | 002254-94-6 |
| benzotriazole, 2-(2-hydroxy-5-methyl-phenyl)- | 002440-22-4 |
| 2-benzothiazolinone | 000934-34-9 |
| benzyl ethyl ether | 000539-30-0 |
| benzyl alcohol, 4-ethoxy | 006214-44-4 |
| benzyl alcohol, alpha, alpha, 4-trimethyl- | 001197-01-9 |
| benzyl alcohol, a,a-dimethyl-p-isopropyl- | 003445-42-9 |
| benzylamine | 000100-46-9 |
| benzyldiphenylphosphine oxide | 002959-74-2 |
| binaphthyl sulfone | 032390-26-4 |
| borneol | 000507-70-0 |
| 1-butanamine,N,N-dibutyl- | 000102-82-9 |
| butanedioic acid | 000110-15-6 |
| butanediol dimethacrylate, 1,4- | 002082-81-7 |
| butanetricarboxylic acid, 2-phosphono-, 1,2,4- | 037971-36-1 |
| butanoic acid | 000107-96-2 |
| butanoic acid, 3,3-dimethyl- | 001070-83-3 |
| buten-1-ol, 2-methyl-2- | 004675-87-0 |
| buten-1-ol, 3-methyl-3- | 000763-32-6 |
| butenal, methyl- | 001115-11-3 |
| butene, 2,3-dichloro-2-methyl- | 000507-45-9 |
| butenoic acid, 2- | 003724-65-0 |
| butenoic acid, 3- | 000625-38-7 |
| butyl isocyanate, n- | 000111-36-4 |
| butylamine, N-butylidene | 004853-56-9 |
| carbodiimide, di-t-butyl- | 000691-24-7 |
| chlorotoluene, p- | 000106-43-4 |
| cinnamate, 2-ethylhexyl-4-methoxy- | 005466-77-3 |
| cyanovaleric acid, 4- | 999900-00-3 |
| cyclododecane | 000294-62-2 |
| cyclohexadecane | 000295-65-8 |
| cyclohexadiene-1-one, 2,6-(1,1-dimethylethyl)-4-methylene-2,5- | 002607-52-5 |
| cyclohexanamine, 4,4'-methylene-bis- | 001761-71-3 |
| cyclohexanamine, N-methyl- | 000100-60-7 |
| cyclohexanamine, N-cyclohexyl- | 000101-83-7 |
| cyclohexanamine, N,N-dimethyl- | 000098-94-2 |
| cyclohexane, cis-1-methyl-4-isopropyl- | 006069-98-3 |
| cyclohexane, 1-isopropyl-4-methyl- | 000099-82-1 |
| cyclohexanemethanol, trans-alpha, alpha,4-trimethyl- | 005114-00-1 |
| cyclohexanol | 000108-93-0 |
| cyclohexanol, 3-methyl- | 000591-23-1 |
| cyclohexanol, trimethyl- | 001321-60-4 |
| cyclohexanol, 4-tert-butyl- | 000098-52-2 |
| cyclohexanone, 2-hydroxy | 000533-60-8 |
| cyclohexanone, 2-(1-hydroxycyclohexyl)- | 028746-99-8 |
| cyclohexen-1-one, 3-methyl-2- | 001193-18-6 |
| cyclohexene, 4-cyano also (1-cyano-3-cyclohexene) | 000100-45-8 D2 |
| cyclohexyl isocyanate | 003173-53-3 |
| cyclohexylurea, dimethyl- | 031468-12-9 |
| cyclopentanone | 000120-92-3 |
| cyclopentylcyclopentanone, 2- | 004884-24-6 |
| decadien-1-al, trans, trans-2,4- | 025152-84-5 |
| decadienal, 2,4- | 002363-88-4 |
| decamethylcyclopentasiloxane | 000541-02-6 |
| decanamide, N,N-dimethyl- | 014433-76-2 |
| decanedioic acid, bis(2,2,6,6-tetramethyl-4-piperidinyl)- | 052829-07-9 |
| dehydroabietic acid | 001740-19-8 |
| di-o-tolylguanidine, 1,3- | 000097-39-2 |
| diazacyclotetradecane-2,9-dione, 1,8- | 056403-09-9 |
| dibenzylamine | 000103-49-1 |
| dibutyl cyanamide, N,N- | 002050-54-6 |
| 1,3-dicyclohexylurea | 002387-23-7 |
| diethylene glycol monomethacrylate homopolymer | 027598-43-2 |
| diethylurea, 1,3- | 000623-76-7 |
| dihydro-5-pentyl-2(3H)-furanone | 000104-61-0 |
| dihydrobenzofuran, 2,3- | 000496-16-2 |
| dihydrofuran, 4-methyl-2,3- | 034314-83-5 |
| dihydromethoxymethyl oxopyridinecarbonitrile | 000524-40-3 |
| dihydromethyl benzimidazolone | 005400-75-9 |
| dimethyl ditallow ammonium chloride | 068783-78-8 |
| dimethyl glutarate | 001119-40-0 |
| dimethyl succinate | 000106-65-0 |
| dimethyl thioacetamide | 000631-67-4 |
| dimethyl-3,3'-thiobispropionate | 004131-74-2 |
| dimethyl-p-benzoquinone, 2,5- | 000137-18-8 |
| dimethylaminopyridine | 001122-58-3 |
| dimethylbenzaldehyde, 2,4- | 015764-16-6 |
| dimethylbenzaldehyde, 2,5 | 005779-94-2 |
| dimethylbenzaldehyde, 3,4- | 005973-71-7 |
| dimethylcyanamide | 001467-79-4 |
| dimethyldiphenyl sulphone | 005097-12-1 |
| dimethyldithiocarbamate, methyl | 003735-92-0 |
| dimethyldodecanamide, N,N- | 003007-53-2 |
| dimethylhexane-2,5-diol, 2,5- | 000110-03-2 |
| dioxacyclododecane-7, 12-dione, 1,6- | 000777-95-7 |
| dioxathiocane, 1,3,6- | 002094-92-0 |
| dioxolane-1,3, 4-ethyl | 029921-38-8 |
| dithiolane-2-thione, 1,3- | 000822-38-8 |
| docosane | 000629-97-0 |
| docosenamide (erucamide) | 000112-84-5 |
| dodecamethylcyclohexasiloxane | 000540-97-6 |
| dodecanamide | 001120-16-7 |
| dodecyl glycidyl ether | 002461-18-9 |
| ethan-1-one, 1-(methylphenyl)- | 026444-19-9 |
| ethane, 1-(3-hydroxyphenyl)-2-phenyl- | 033675-75-1 |
| ethanediamide, N-(2-ethoxyphenyl)-N'-(2-ethylphenyl)- | 023949-66-8 D3 |
| ethanol, 2-[2-[2-[2[(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethoxy]- | 049796-75-0 |
| ethanol, 2-[2-[2-[(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethoxy]- | 058705-51-4 |
| ethanol, 2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]- | 002315-61-9 |
| ethanone, 1-(4-hydroxy-3-methoxyphenyl)- | 000498-02-2 |
| ethanone, 1-(4-(1-hydroxy-1-methylethyl)phenyl)- | 054549-72-3 |
| ethyl hydroxyphthalide | 000485-26-7 |
| ethylcyclopentanone | 004971-18-0 |
| ethylene glycol dimethacrylate | 000097-90-5 |
| fenchyl alcohol | 001632-73-1 |
| fenchyl alcohol, alpha- | 000512-13-0 |
| fenchyl alcohol, alpha- | 014575-74-7 |
| fluorenone | 000486-25-9 |
| formamide, N,N-diethyl- | 000617-84-5 |
| formamide, N-methyl-N-phenyl- | 000093-61-8 |
| formamide, N-cyclohexyl- | 000766-93-8 |
| formamide, N-(1, 1-dimethylethyl)- | 002425-74-3 |
| formamide, N,N-di-n-butyl- | 000761-65-9 |
| formamidine, N,N-dimethyl-N'-cyclohexyl- | 003459-75-4 |
| formylcyclopentene, 1- | 006140-65-4 |
| furan, tetrahydro-2,2,5,5-tetramethyl- | 015045-43-9 |
| furfural, 5-methyl | 000620-02-0 |
| geraniol | 000106-24-1 |
| guanidine, 1,2,3-triphenyl- | 000101-01-9 |
| heptacosane | 000593-49-7 |
| heptyl aldehyde, n- | 000111-71-7 |
| hexacosane | 000630-01-3 |
| hex-1-ene, 2-ethyl- | 001632-16-2 |
| hex-5-en-1-ol | 000821-41-0 |
| hexadecanamide | 000629-54-9 |
| hexadecanamide, N,N-dimethyl- | 003886-91-7 |
| hexadecene-1 | 000629-73-2 |
| hexamethylene oxide | 000592-90-5 |
| hexamethylene dibenzamide | 005326-21-6 |
| hexanal, 2-ethyl- | 000123-05-7 |
| hexanal | 000066-25-1 |
| hexanamine, 2- | 005329-79-3 |
| hexane, 2,5-dimethyl- | 000592-13-2 |
| hexane-2,5-dione | 000110-13-4 |
| hexaoxacyclotriacontane, 1,6,11,16,21,26- | 064001-05-4 |
| hexen-2-one, 5-methyl-5- | 003240-09-3 |
| hydrocinnamic acid | 006386-38-5 |
| hydroxydiphenylamine, 3- | 000101-18-8 |
| hydroxypropyl methacrylate, 2- | 000923-26-2 |
| icosane | 000112-95-8 |
| imidazole, methylphenyl- | 000670-91-7 |
| Indan-1-one | 000083-33-0 |
| indene, 2,3-dihydro- also (2,3-dihydro-1H-) | 000496-11-7 |
| indene | 000095-13-6 |
| isobutyramide | 000563-83-7 D4 |
| isocrotonic acid | 000503-64-0 |
| isophorone diamine | 002855-13-2 |
| isovanillin | 000621-59-0 |
| laurolactam | 000947-04-6 |
| methacrylate, lauryl- | 000142-90-5 |
| methacrylic acid | 000079-41-4 |
| methacrylic acid, 3-(trimethylsilyl)propyl ester | 002530-85-0 |
| methane, di-t-butyl- | 001070-87-7 |
| methoxybenzene | 000100-66-3 |
| methyl anthranilate | 000134-20-3 |
| methyl palmitate | 000112-39-0 |
| methyl laurate | 000111-82-0 |
| methyl stearate | 000112-68-1 |
| methyl-4-isopropyl cyclohexane, trans-1- | 001678-82-6 |
| methyldiethyl carbamate | 004652-44-2 |
| methylene bis(4-methyl-6-tertbutyl-phenol), 2, 2' | 000119-47-1 |
| 2,2'-methylenediphenol | 002467-02-9 |
| 4,4'-methylenediphenol | 000620-92-8 |
| methylenephenethyl alcohol, beta- | 006006-81-1 |
| methylindene | 029036-25-7 |
| methylpiperidine, 1- | 000626-67-5 |
| methylthioacetonitrile | 035120-10-6 |
| morpholine, methyl- | 000109-02-4 |
| morpholinecarbaldehyde, 4- | 004394-85-8 |
| morpholinecarboxamide, N-cyclohexyl-4- | 003417-54-7 |
| morpholinepropanenitrile, 4- | 004542-47-6 |
| N-butyl formamide | 000871-71-6 |
| N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate | 000078-44-4 |
| naphthalene, dimethyl- | 028804-88-8 |
| naphthalene, ethyl | 027138-19-8 |
| noanoic acid, n- | 000112-05-0 |
| nonanal | 000124-19-6 |
| nonanoic acid, 9-oxo- | 002553-17-5 |
| norbornene, 5-ethylidene-2- | 016219-75-3 |
| octadecadienoic acid, (Z,Z)-9, 12-, butyl ester | 002634-45-9 |
| octadecanamide | 000124-26-5 |
| octadecenamide | 000301-02-0 |
| octadecene, 1- | 000112-88-9 |
| octadien-1-ol, 3,7-dimethyl-2,6- | 000624-15-7 |
| octadien-2-ol, 2,6-dimethyl-5,7- | 005986-38-9 |
| octadien-3-ol, 2,6-dimethyl-1,7- | 022460-59-9 |
| octadien-3-ol, 3,7-dimethyl-1,6- | 000078-70-6 |
| octadien-3-ol, 3,7-dimethyl-4,6- | 018479-54-4 |
| octanal | 000124-13-0 |
| octanoate, methyl- | 000111-11-5 |
| octaphenyl pentaethylene glycol ether, tert- | 038621-31-7 |
| Octen-3-ol, 1- | 003391-86-4 |
| octylphenoxypentaethoxyethanol, tert- | 037809-81-7 |
| oleate, n-butyl- | 000142-77-8 D5 |
| oxabicyclo (4.1.0) heptane-3-carboxylic acid, 7- | 002386-87-0 |
| oxamide, di-tert-butyl- | 037486-48-9 |
| oxaspirodecadienedione, di-(t-butyl) | 082304-66-3 |
| oxirane, [(dodecyloxy)methyl]- | 002461-18-9 |
| oxybis(propanenitrile) | 001656-48-0 |
| palmitate, isopropyl- | 000142-91-6 |
| palmitic acid, n-butyl ester | 000111-06-8 |
| pentacosane | 000629-99-2 |
| pentane, 1-amino | 000110-58-7 |
| pentanediol, 2,2,4-trimethyl-1,3- | 000144-19-4 |
| pentanenitrile | 000110-59-8 |
| pentaoxacyclopentacosane, 1,6,11,16,21,- | 056890-57-4 |
| pentenal, trans-2- | 001576-87-0 |
| peroxide, tert-butyl- | 000110-05-4 |
| phenanthrene | 000085-01-8 |
| phenol, 4-ethoxy- | 000622-62-8 |
| phenol, o-(1-phenylethyl)- | 004237-44-9 |
| phenol, (phenylethyl)- | 051937-33-8 |
| phenol, o-(alpha, alpha-dimethylbenzyl)- | 018168-40-6 |
| phenol, p-(alpha, alpha-dimethylbenzyl)- | 000599-64-4 |
| phenol, p-phenylethyl- | 006335-83-7 |
| phenol, 4-(2-propenyl)- | 000501-92-8 |
| phenol, 3,5-dibenzyl-2,4,6-trimenthyl- | 999900-00-1 |
| phenol, 2,6-di-t-butyl-4-methyl- | 000489-01-0 |
| phenol, 4-(1-phenylethyl)- | 001988-89-2 |
| phenol isothiocyanate | 000103-72-0 |
| phenyl-1-buten-4-ol, 4- | 000936-58-3 |
| phenylbutane, 2- | 000135-98-8 |
| phenylene) bis-ethanone, 1,1'-(1,4- | 001009-61-6 |
| phenylene) bis-ethanone, 1,1'-(1,3- | 006781-42-6 |
| 2,2'-p-phenylenedioxydiethanol | 000104-38-1 |
| phenylethanol, 2- | 000060-12-8 |
| (phenylimino) cyclohexadiene | 002406-04-4 |
| phenylindan, 1,1,3-trimethyl-3- | 003910-35-8 |
| phosphate, diphenyl-2-ethylhexyl- | 001241-94-7 |
| phosphonic acid, (nitrilotris(methylene))tri-, pentasodium | 002235-43-0 |
| phthalide [also 1(3H)-isobenzofuranone] | 000087-41-2 |
| pinanol | 000473-54-1 |
| pinanol (or cis-2-pinanol) | 004948-28-1 |
| pinocampheol (also pinocamphone) | 000547-60-4 |
| piperidine, 1-formyl | 002591-86-8 |
| piperidine, 2-propyl- | 000458-88-8 |
| piperidinol, 1,2,2,6,6-pentamethyl-4- | 002403-89-6 |
| poly(oxy-1,2-ethanediyl), a-isotridecyl-w-hydroxy-, phosphate | 073038-25-2 |
| propanal, 2,2-dimethyl-3-hydroxy- | 000597-31-9 |
| propanaminium chloride, N,N,N-trimethyl-3-((1-oxo-2-propenyl)amino)-1- | 045021-77-0 |
| propane, 1,1-dimethoxy-2-methyl | 041632-89-7 |
| propanediol, 2-ethyl-2-butyl-1,3- | 000115-84-4 D6 |
| propanenitrile, 3,3'-oxybis- | 001656-48-0 |
| propanenitrile, 3,3'-thiobis- | 000111-97-7 |
| propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester | 074381-40-1 |
| propanoic acid, 3-ethoxy-, ethyl ester | 000763-69-9 |
| propanoic acid, 2,2-dimethyl- | 000075-98-9 |
| propanoic acid, 2-methyl-, 3-hydroxy-2,4,4-trimethylpentyl ester | 000077-68-9 |
| propanoic acid, 2-methyl-, 3-hydroxy-2,4,4-trimethylpentyl ester | 074367-34-3 |
| propanoic acid, 2-methyl, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)propyl ester | 074367-33-2 |
| propanol, 1-amino-2- | 000078-96-6 |
| propanol, phenyl-1- | 001335-12-2 |
| propanol, 1-propoxy-2- | 001569-01-3 |
| propanone, 1-phenyl-1- | 000093-55-0 |
| propenoic acid, 2-methyl-2-, polymer with octadecyl-2-methyl-2-propenoate | 027401-06-5 |
| propenone, (dihydroxy methoxyphenyl) phenyl- | 018956-15-5 |
| pyrene | 000129-00-0 |
| pyridine, 2,4,-dimethyl- | 000108-47-4 |
| pyridine, 2,4,6-trimethyl- | 000108-75-8 |
| pyridine, 1,2,3,4-tetrahydro-1,2,2,6-tetramethyl- | 063867-76-5 |
| pyridine, 1,2,3,6-tetrahydro-1,2,3,4-tetramethyl- | 090949-18-1 |
| pyridine, 1,2,3,6-tetrahydro-1,2,4,5-tetramethyl- | 090949-19-2 |
| pyridine, 1,2,3,6-tetrahydro-1,2,4,6-tetramethyl-, cis- | 023513-16-8 |
| pyridine, 1,2,3,6-tetrahydro-1,3,3,6-tetramethyl- | 122913-54-6 |
| pyridine, 1,2,3,6-tetrahydro-1,4,5,6-tetramethyl- | 090949-20-5 |
| pyridine, 1,2,3,6-tetrahydro-2,2,2,6-tetramethyl- | 001124-69-2 |
| pyridine, 1,2,5,6-tetrahydro-2,2,5,5-tetramethyl- | 155904-89-5 |
| pyridine, 2,3,4,5-tetrahydro-2,2,4,6-tetramethyl- | 200561-41-7 |
| pyrrolidine | 000123-75-1 |
| sodium p-sulfophenyl methallyl ether | 001208-67-9 |
| squalene | 007683-64-9 |
| styrene, alpha-methyl- | 000098-83-9 |
| styrene, methyl- (mixed isomers) | 025013-15-4 |
| sulfonylbis(4-methyl)-benzene, 1,' | 000599-66-6 |
| terephthalic acid, monomethyl ester | 001679-64-7 |
| terpineol, alpha- | 000098-55-5 |
| tert-butylamine | 000075-64-9 |
| tetracosane | 000646-31-1 |
| tetradecamethylcycloheptasiloxane | 000107-50-6 |
| tetradecanamide | 000638-58-4 |
| tetradecane | 001120-36-1 |
| tetraethyleneglycol di-(2-ethylhexoate) | 018268-70-7 |
| tetraethyleneglycol dimethacrylate | 000109-17-1 |
| tetrahydrofuran, diphenyl- | 050637-09-7 |
| tetrahydropyridine, 2,3,4,5- | 000505-18-0 |
| tetramethyl urea | 000632-22-4 |
| tetramethyldec-5-yne-4,7-diol, 2,4,7,9- | 000126-86-3 |
| tetramethyldecynediol | 001333-17-1 |
| 2,6,10,14-tetramethylhexadecane | 000638-36-8 D7 |
| tetramethylpyrazine, 2,3,5,6- | 001124-11-4 |
| tetramethylsuccinonitrile | 003333-52-6 |
| tetraoxacycloeicosane, 1,6,11,16- | 017043-02-6 |
| tetrathiacyclooctadecane, 1,3,10,12-tetraoxa-6,7,15,16- | 099634-55-6 |
| 4,4'-thiobis-(6-t-butyl-o-cresol) | 000096-66-2 |
| triallyl cyanurate | 000101-37-1 |
| tributoxyethyl phosphate | 000078-51-3 |
| tributylphosphine oxide | 000814-29-9 |
| trichlorotrifluoroethane | 026523-64-8 |
| Tricosane, also (n-tricosane) | 000638-67-5 |
| triethylamine | 000121-44-8 |
| triethyleneglycol dimethacrylate | 000109-16-0 |
| triethylsilanol | 000597-52-4 |
| trimethylcyclohexanone | 050874-76-5 |
| trimethylolpropane trimethacrylate | 003290-92-4 |
| trioxepane | 005981-06-6 |
| triphenylphosphate | 000115-86-6 |
| triphenylphosphine oxide | 000791-28-6 |
| urea, N,N-bis-(1,1-dimethylethyl)- | 005336-24-3 |
| valeronitrile, 2,4-dimethyl- | 034372-09-3 |
| 1 For the chemicals listed in this table, the evaluation criteria are 0.003 mg/L under static conditions, and 0.0003 mg/L under flowing conditions. | |
Annex E3
| Substance | CAS # |
|---|---|
| Inorganics | |
| cobalt | 7440-48-4 |
| sodium thiocyanate | 540-72-7 |
| titanium | 7440-32-6 |
| Organics | |
| benzene, 1-chloro-4-(trifluoromethyl)- | 98-56-6 |
| benzamide | 55-21-0 |
| benzoguanamine | 91-76-9 |
| benzotriazole, 1,2,3- | 95-14-7 |
| benzyl acetate | 140-11-4 |
| benzyl alcohol, 3,5-di-tert-butyl-4-hydroxy- | 88-26-6 |
| butyl acetate | 123-86-4 |
| cyanoguanidine | 461-58-5 |
| cyclohexene | 110-83-8 |
| dichlorodifluoromethane | 75-71-8 |
| dichlorophenol, 2,4- | 120-83-2 |
| diethylaminoethanol | 100-37-8 |
| dimethylacetamide, n,n- | 127-19-5 |
| dimethyl adipate | 627-93-0 |
| dimethylaminopropanenitrile | 1738-25-6 |
| dimethylformamide, n,n- | 68-12-2 |
| dimethyl phthalate | 131-11-3 |
| diphenyl guanidine, 1,3- (or n,n-) | 102-06-7 |
| diphenyl-p-phenylenediamine, n,n'- | 74-31-7 |
| ethanol, 2-diethylamino- | 100-37-8 |
| ethanol, 2-(dimethylamino)- | 108-01-0 |
| ethanol, 2-phenoxy- | 122-99-6 |
| ethanol, 1-phenyl- | 98-85-1 |
| fluoranthene | 206-44-0 |
| furanmethanol, 2- | 98-00-0 |
| heptanoic acid, n- | 111-14-8 |
| hexamethylenediamine | 124-09-4 |
| hexamethylenetetramine | 100-97-0 |
| hexanoic acid, n- | 142-62-1 |
| hexanoic acid, 2-ethyl | 149-57-5 E1 |
| isobutyl isobutyrate | 97-85-8 |
| (isopropylamino)diphenylamine, 4- | 101-72-4 |
| isopropyltoluene | 99-87-6 |
| methyl acrylate | 96-33-3 |
| Organics | |
| methyldiethanolamine, n- | 105-59-9 |
| methylene diiphenyl disocyanate | 101-68-8 |
| methylene bis(n-iso-butylbenzenamine) | 88990-59-4 |
| phenylene diamine, n-(1,3-dimethylbutyl)-n‘-phenyl-p- | 793-24-8 |
| phenylenediamine, n-phenyl-p- | 101-54-2 |
| phthalic acid, o- | 88-99-3 |
| piperidine | 110-89-4 |
| sebacate, bis(2-ethylhexyl)- | 122-62-3 |
| silane, gamma-aminopropyl triethoxy- | 919-30-2 |
| tacrine | 321-64-2 |
| tetramethylene sulfone | 126-33-0 |
| tetramethyl piperidinone | 826-36-8 |
| triallyl isocyanurate | 1025-15-6 |
| triethylene diamine | 280-57-9 |
| tris(2-ethylhexyl) phosphate | 78-42-2 |
| vanillin, o- | 148-53-8 |
| 3 The information contained in this Annex is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI’s requirements for an ANS. As such, this Annex may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements necessary for conformance to the Standard. 1 For the chemicals in this table, the evaluation criteria are 0.003 mg/L under static conditions and 0.0003 mg/L under flowing conditions. The chemicals that appear in this table have been detected only at concentrations not exceeding these threshold of evaluation concentrations as established in this standard (see annex A, A.7.1), and have not been evaluated for specific TAC and SPAC values. If any of these chemicals are detected at concentrations exceeding the threshold of evaluation, toxicity data shall be reviewed to determine whether specific TAC and SPAC values can be established, prior to using threshold of evaluation to determine compliance with the Standard. |
|
Standards and Criteria4
The following standards and criteria established and adopted by NSF as minimum voluntary consensus standards are used internationally:
2 Food equipment
3 Commercial warewashing equipment
4 Commercial cooking, rethermalization, and powered hot food holding and transport equipment
5 Water heaters, hot water supply boilers, and heat recovery equipment
6 Dispensing freezers
7 Commercial refrigerators and freezers
8 Commercial powered food preparation equipment
12 Automatic ice making equipment
13 Refuse processors and processing systems
14 Plastics piping system components and related materials
18 Manual food and beverage dispensing equipment
20 Commercial bulk milk dispensing equipment
21 Thermoplastic refuse containers
24 Plumbing system components for manufactured homes and recreational vehicles
25 Vending machines for food and beverages
29 Detergent and chemical feeders for commercial spray-type dishwashing machines
35 High pressure decorative laminates (HPDL) for surfacing food service equipment
36 Dinnerware
37 Air curtains for entranceways in food and food service establishments
40 Residential wastewater treatment systems
41 Non-liquid saturated treatment systems
42 Drinking water treatment units — Aesthetic effects
44 Residential cation exchange water softeners
46 Evaluation of components and devices used in wastewater treatment systems
49 Class II (laminar flow) biohazard cabinetry
50 Circulation system components and related materials for swimming pools, spas/hot tubs
51 Food equipment materials
52 Supplemental flooring
53 Drinking water treatment units – Health effects
55 Ultraviolet microbiological water treatment systems
58 Reverse osmosis drinking water treatment systems
59 Mobile food carts
60 Drinking water treatment chemicals — Health effects
61 Drinking water system components — Health effects
62 Drinking water distillation systems
75 Non-potentially hazardous foods
116 Non-food compounds used in food processing facilities — Food grade lubricants (draft standard for trial use)
173 Dietary supplements (draft standard for trial use)
184 Residential dishwashers
14159 Safety of machinery — Hygiene requirements for the design of machinery
14159-1 Hygiene requirements for the design of meat and poultry processing equipment C-2 Special equipment and/or devices
4 The information contained in this Standards and Criteria page is not part of this American National Standard (ANS) and has not been processed in accordance with ANSI’s requirements for an ANS. As such, this Standards and Criteria page may contain material that has not been subjected to public review of a consensus process. In addition, it does not contain requirements snecessary for conformance to the Standard.
E3 E4
THE HOPE OF MANKIND rests in the ability of man to define and seek out the environment which will permit him to live with fellow creatures of the earth, in health, in peace, and in mutual respect.
Printed 01/15/02
E5