In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
18TH EDITION 1992
Prepared and published jointly by:
AMERICAN PUBLIC HEALTH ASSOCIATION
AMERICAN WATER WORKS ASSOCIATION
WATER ENVIRONMENT FEDERATION
Joint Editorial Board
Arnold E. Greenberg, APHA, Chairman
Lenore S. Clesceri, WEF
Andrew D. Eaton, AWWA
Managing Editor
Mary Ann H. Franson
Publication Office
American Public Health Association
1015 Fifteenth Street, NW
Washington, DC 20005
Copyright © 1917, 1920, 1923, and 1925 by
American Public Health Association
Copyright © 1933, 1936, and 1946 by
American Public Health Association
American Water Works Association
Copyright © 1955, 1960, and 1965 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1971 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1976 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1981 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1985 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1989 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1992 by
American Public Health Association
American Water Works Association
Water Environment Federation.
All rights reserved. No part of this publication may be reproduced, graphically or electronically, including entering in storage or retrieval systems, without the prior written permission of the publishers.
30M7/92
The Library of Congress has catalogued this work as follows:
American Public Health Association.
Standard methods for the examination of water and wastewater.
ISBN 0-87553-207-1
Printed and bound in the United States of America.
Composition: EPS Group, Inc., Hanover, Maryland
Set in: Times Roman
Printing: Victor Graphics, Inc., Baltimore, Maryland
Binding: American Trade Bindery, Baltimore, Maryland
Cover Design: DR Pollard and Associates, Inc., Arlington, Virginia
Because the physical and chemical properties of chlorine dioxide resemble those of chlorine in many respects, read the entire discussion of Residual Chlorine (Section 4500-Cl) before attempting a chlorine dioxide determination.
Chlorine dioxide, ClO2, has been used widely as a bleaching agent in the paper and pulp industry. It has been applied to water supplies to combat tastes and odors due to phenolic-type wastes, actinomycetes, and algae, as well as to oxidize soluble iron and manganese to a more easily removable form. It is a disinfectant, and some results suggest that it may be stronger than free chlorine or hypochlorite.
Chlorine dioxide is a deep yellow, volatile, unpleasant-smelling gas that is toxic and under certain conditions may react explosively. It should be handled with care in a vented area. There are several methods of generating ClO2; for laboratory purposes the acidification of a solution of sodium chlorite followed by suitable scrubbing is the most practical.
The iodometric method (B) gives a very precise measure of total available strength of a solution in terms of its ability to liberate iodine from iodide. However, ClO2, chlorine, chlorite, and hypochlorite are not distinguished easily by this technique. It is designed primarily, and best used, for standardizing ClO2 solutions needed for preparation of temporary standards. It often is inapplicable to industrial wastes.
The amperometric methods (C and E) are useful when a knowledge of the various chlorine fractions in a water sample is desired. They distinguish various chlorine compounds of interest with good accuracy and precision, but require specialized equipment and considerable analytical skill.
The N, N-diethyl-p-phenylenediamine (DPD) method (D) has the advantages of a relatively easy-to-perform colorimetric test with the ability to distinguish between ClO2 and various forms of chlorine. This technique is not as accurate as the amperometric method, but should yield results adequate for most common applications.
* Approved by Standard Methods Committee, 1988.
4-53Determine ClO2 promptly after collecting the sample. Do not expose sample to sunlight or strong artificial light and do not aerate to mix. Minimum ClO2 losses occur when the determination is completed immediately at the site of sample collection.
INGOLS, R.S. & G.M. RIDENOUR, 1948. Chemical properties of chlorine dioxide in water treatment. J. Amer, Water Works Assoc. 40:1207.
PALIN, A.T. 1948. Chlorine dioxide in water treatment. J. Inst. Water Eng. 11:61.
HODGDEN, H.W. & R.S. INGOLS. 1954. Direct colorimetric method for determination of chlorine dioxide in water. Anal. Chem. 26:1224.
FEUSS, J.V. 1964. Problems in determination of chlorine dioxide residuals. J. Amer. Water Works Assoc. 56:607.
MASSCHELEIN, W. 1966. Spectrophotometric determination of chlorine dioxide with acid chrome violet K, Anal. Chem. 38:1839.
MASSCHELEIN, W. 1969. Les Oxydes de Chlore et le Chlorite de Sodium, Dunod, Paris, Chapter XI.
a. Principle: A pure solution of ClO2 is prepared by slowly adding dilute H2SO4 to a sodium chlorite (NaClO2) solution. Contaminants such as chlorine are removed by a NaClO2 scrubber and passing the gas into distilled water in a steady stream of air.
ClO2 releases free iodine from a KI solution acidified with acetic acid or H2SO4. The liberated iodine is titrated with a standard solution of sodium thiosulfate (Na2S2O3), with starch as the indicator.
b. Interference: There is little interference in this method, but temperature and strong light affect solution stability. Minimize ClO2 losses by storing stock ClO2 solution in a dark refrigerator and by preparing and titrating dilute ClO2 solutions for standardization purposes at the lowest practicable temperature and in subdued light.
c. Minimum detectable concentration: One drop (0.05 mL) of 0.01N Na2S2O3 is equivalent to 20 µg ClO2/L (or 40 µg/L in terms of available chlorine) when a 500-mL sample is titrated.
All reagents listed for the determination of residual chlorine in Section 4500-Cl.B.2a-g are required. Also needed are the following:
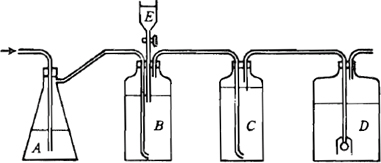
a. Stock chlorine dioxide solution: Prepare a gas generating and absorbing system as illustrated in Figure 4500-ClO2:1. Connect aspirator flask (A), 500-mL capacity, with rubber tubing to a source of compressed air. Let air bubble through a layer of 300 mL distilled water in flask and then pass through a glass tube ending within 5 mm of the bottom of the 1-L gas-generating bottle (B). Conduct evolved gas via glass tubing through a scrubber bottle (C) containing saturated NaClO2 solution or a tower packed with flaked NaClO2 and finally, via glass tubing, into a 2-L borosilicate glass collecting bottle (D) where the gas is absorbed in 1500 mL distilled water. Provide an air outlet tube on collecting bottle (D) for escape of air. Select for gas generation a bottle constructed of strong borosilicate glass and having a mouth wide enough to permit insertion of three separate glass tubes: the first leading almost to the bottom for admitting air, the second reaching below the liquid surface for gradual introduction of H2SO4, and the third near the top for exit of evolved gas and air. Fit to second tube a graduated cylindrical separatory funnel (E) to contain H2SO4. Locate this system in a fume hood with an adequate shield.

Figure 4500-ClO2:1. Chlorine dioxide generation and absorption system.
Dissolve 10 g NaClO2 in 750 mL distilled water and place in generating bottle (B). Carefully add 2 mL cone H2SO4 to 18 mL, distilled water and mix. Transfer to funnel. Connect flask to generating bottle, generating bottle to scrubber, and the latter to collecting bottle. Pass a smooth current of air through the system, as evidenced by the bubbling rate in all bottles.
Introduce 5-mL increments of H2SO4 from funnel into generating bottle at 5-min intervals. Continue air flow for 30 min after last portion of acid has been added.
Store yellow stock solution in glass-stoppered dark-colored bottle in a dark refrigerator. The concentration of ClO2 thus prepared varies between 250 and 600 mg/L, corresponding to approximately 500 to 1200 mg free chlorine/L.
b. Standard chlorine dioxide solution: Use this solution for preparing temporary ClO2 standards. Dilute required volume of stock ClO2 solution to desired strength with chlorine-demand-free water (see Section 4500-Cl.C.3m). Standardize solution by titrating with standard 0.01N or 0.025N Na2S2O3 titrant in the presence of KI, acid, and starch indicator by following the procedure given in ¶ 3 below. A full or nearly full bottle of chlorine or ClO2 solution retains its titer longer than a partially full one. When repeated withdrawals reduce volume to a critical level, standardize diluted solution at the beginning, midway in the series of withdrawals, and at the end of the series. Shake contents thoroughly before drawing off needed solution from middle of the glass-stoppered dark-colored bottle. Prepare this solution frequently.
4-54Select volume of sample, prepare for titration, and titrate sample and blank as described in Section 4500-Cl.B.3. The only exception is the following: Let ClO2 react in the dark with acid and KI for 5 min before starting titration.
Express ClO2 concentrations in terms of ClO2 or as free chlorine content. Free chlorine is defined as the total oxidizing power of ClO2 measured by titrating iodine released by ClO2 from an acidic solution of KI. Calculate result in terms of chlorine itself.
For standardizing ClO2 solution:
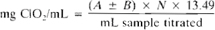
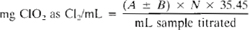
For determining ClO2 temporary standards:
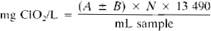
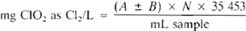
where:
A = mL titration for sample.
B = mL titration for blank (positive or negative, see 4500-Cl.B.3d), and
N = normality of Na2S2O3.
POST, M. A. & W. A. MOORE. 1959. Determination of chlorine dioxide in treated surface waters. Anal. Chem. 31:1872.
a. Principle: The amperometric titration of ClO2 is an extension of the amperometric method for chlorine. By performing four titrations with phenylarsine oxide, free chlorine (including hypochlorite and hypochlorous acid), chloramines, chlorite, and ClO2 may be determined separately. The first titration step consists of conversion of ClO2 to chlorite and chlorate through addition of sufficient NaOH to produce a pH of 12, followed by neutralization to a pH of 7 and titration of free chlorine. In the second titration KI is added to a sample that has been treated similarly with alkali and had the pH readjusted to 7; titration yields free chlorine and monochloramine. The third titration involves addition of KI and pH adjustment to 7, followed by titration of free chlorine, monochloramine, and one-fifth of the available ClO2. In the fourth titration, addition of sufficient H2SO4 to lower the pH to 2 enables all available ClO2 and chlorite, as well as the total free chlorine, to liberate an equivalent amount of iodine from the added KI and thus be titrated.
b. Interference: The interferences described in Section 4500-Cl.D.1b apply also to determination of ClO2.
The apparatus required is given in Sections 4500-Cl.D.2a through d.
All reagents listed for the determination of chlorine in Section 4500-Cl.D.3 are required. Also needed are the following:
a. Sodium hydroxide, NaOH, 6N.
b. Sulfuric acid, H2SO4, 6N, 1+ 5.
Minimize effects of pH, time, and temperature of reaction by standardizing all conditions.
a. Titration of free available chlorine (hypochlorite and hypochlorous acid): Add sufficient 6N NaOH to raise sample pH to 12. After 10 min, add 6N H2SO4 to lower pH to 7. Titrate with standard phenylarsine oxide titrant to the amperometric end point as given in Section 4500-Cl.D. Record result as A.
b. Titration of free available chlorine and chloramine: Add 6N NaOH to raise sample pH to 12. After 10 min, add 6N H2SO4 to reduce pH to 7. Add 1 mL KI solution. Titrate with standard phenylarsine oxide titrant to the amperometric end point. Record result as B.
c. Titration of free available chlorine, chloramine, and one-fifth of available ClO2. Adjust sample pH to 7 with pH 7 phosphate buffer solution. Add 1 mL KI solution. Titrate with standard phenylarsine oxide titrant to the amperometric end point. Record result as C.
d. Titration of free available chlorine, chloramines, ClO2, and chlorite: Add 1 mL KI solution to sample. Add sufficient 6N H2SO4 to lower pH to 2. After 10 min, add sufficient 6NNaOH to raise pH to 7. Titrate with standard phenylarsine oxide titrant to the amperometric end point. Record result as D.
Convert individual titrations (A, B, C and D) into chlorine concentration by the following equation:
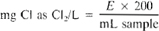
where:
E = mL phenylarsine oxide titration for each individual sample A, B, C, or D.
Calculate ClO2 and individual chlorine fractions as follows:
mg ClO2 as ClO2/L = 1.9 (C − B)
mg ClO2 as Cl2/L = 5 (C − B)
mg free available chlorine/L = A
mg chloramine/L as chlorine = B − A
mg chlorite/L as chlorine = 4B − 5C+ D
HALLER, J. F. & S. S. LISTEK. 1948. Determination of chlorine dioxide and other active chlorine compounds in water. Anal. Chem. 20:639.
a. Principle; This method is an extension of the N, N-diethyl-p-phenylenediamine (DPD) method for determining free chlorine and chloramines in water. ClO2 appears in the first step of this procedure but only to the extent of one-fifth of its total available chlorine content corresponding to reduction of ClO2 to chlorite ion. If the sample is then acidified in the presence of iodide the chlorite also reacts. When neutralized by subsequent addition of bicarbonate, the color thus produced corresponds to the total available chlorine content of the ClO2. If chlorite is present in the sample, this will be included in the step involving acidification and neutralization. Chlorite that did not result from ClO2 reduction by the procedure will cause a positive error equal to twice this chlorite concentration. In evaluating mixtures of these various chloro-compounds. it is necessary to suppress free chlorine by adding glycine before reacting the sample with DPD reagent. Differentiation is based on the fact that glycine converts free chlorine instantaneously into chloroaminoacetic acid but has no effect on ClO2.
b. Interference: The interference by oxidized manganese described in Section 4500-Cl.F.1d applies also to ClO2 determination. Manganese interference appears as an increase in the first titrations after addition of DPD, with or without KI, and irrespective of whether there has been prior addition of glycine. Titration readings must be corrected suitably. Interference by chromate in wastewaters may be corrected similarly.
Iron contributed to the sample by adding ferrous ammonium sulfate (FAS) titrant may activate chlorite so as to interfere with the first end point of the titration. Suppress this effect with additional EDTA, disodium salt.
Reagents required in addition to those for the DPD free-combined chlorine method as listed in Section 4500-Cl.F.2 are as follows:
a. Glycine solution: Dissolve 10 g NH2CH2COOH in 100 mL distilled water.
b. Sulfuric acid solution: Dilute 5 mL conc H2SO4 to 100 mL with distilled water.
c. Sodium bicarbonate solution: Dissolve 27.5 g NaHCO3 in 500 mL distilled water.
d. EDTA: Disodium salt of ethylenediamine tetraacetic acid, solid.
For samples containing more than 5 mg/L total available chlorine follow the dilution procedure given in Section 4500-Cl.F.3.
a. Chlorine dioxide: Add 2 mL glycine solution to 100 mL sample and mix. Place 5 mL each of buffer reagent and DPD indicator solution in a separate titration flask and mix (or use about 500 mg DPD powder). Add about 200 mg EDTA. disodium salt. Then add glycine-treated sample and mix. Titrate rapidly with standard FAS titrant until red color is discharged (Reading G).
b. Free available chlorine and chloramine: Using a second 100-mL sample follow the procedures of Section 4500-Cl.F.3a adding about 200 mg EDTA, disodium salt, initially with the DPD reagents (Readings A, B, and C).
c. Total available chlorine including chlorite: After obtaining Reading C add 1 mL H2SO4 solution to the same sample in titration flask, mix, and let stand about 2 min. Add 5 mL NaHCO3 solution, mix, and titrate (Reading D).
d. Colorimetric procedure: Instead of titration with standard FAS solution, colorimetric procedures may be used to obtain the readings at each stage. Calibrate colorimeters with standard permanganate solution as directed in Section 4500-Cl.G.4a. Use of additional EDTA, disodium salt, with the DPD reagents is not required in colorimetric procedures.
For 100 mL sample, 1 mL FAS solution = 1 mg available chlorine/L.
In the absence of chlorite:
Chlorine dioxide = 5G (or 1.9G expressed as ClO2)
Free available chlorine = A − G
Monochloramine = B − A
Dichloramine = C − B
Total available chlorine = C + 4G
If the step leading to Reading B is omitted, monochloramine and dichloramine are obtained together when:
Combined available chlorine = C − A
4-56If it is desired to check for presence of chlorite in sample, obtain Reading D. Chlorite is indicated if D is greater than C + 4G.
In the presence of chlorite:
Chlorine dioxide = 5G (or 1.9G expressed as ClO2)
Chlorite = D −(C + 4G)
Free available chlorine = A − G
Monochloramine = B − A
Dichloramine = C − B
Total available chlorine = D
If B is omitted,
Combined available chlorine = C − A
PALIN, A.T. 1960. Colorimetric determination of chlorine dioxide in water. Water Sewage Works 107:457.
PALIN, A.T. 1967. Methods for the determination, in water, of free and combined available chlorine, chlorine dioxide and chlorite, bromine, iodine, and ozone using diethyl-p-phenylenediamine (DPD). J. Inst. Water Eng. 21:537.
PALIN, A.T. 1974. Analytical control of water disinfection with special reference to differential DPD methods for chlorine, chlorine dioxide, bromine, iodine and ozone. J. Inst. Water Eng. 28:139.
PALIN, A. T. 1975. Current DPD methods for residual halogen compounds and ozone in water. J. Amer. Water Works Assoc. 67:32.
a. Principle: Like Amperometric Method I (Section 4500-ClO2.C), this procedure entails successive titrations of combinations of chlorine species. Subsequent calculations determine the concentration of each species. The equilibrium for reduction of the chlorine species of interest by iodide is pH-dependent.
The analysis of a sample for chlorine, chlorine dioxide, chlorite, and chlorate required the following steps: determination of all of the chlorine (free plus combined) and one-fifth of the chlorine dioxide at pH 7; lowering sample pH to 2 and determination of the remaining four-fifths of the ClO2 and all of the chlorite (the chlorite measured in this step comes from the chlorite originally present in the sample and that formed in the first titration); preparation of a second sample by purging with nitrogen to remove ClO2 and by reacting with iodide at pH 7 to remove any chlorine remaining; lowering latter sample pH to 2 and determination of all chlorite present (this chlorite only comes from the chlorite originally present in the sample); and, in a third sample, determination of all of the relevant, oxidized chlorine species—chlorine, chlorine dioxide, chlorite, and chlorate—after reduction in hydrochloric acid.1
This procedure can be applied to concentrated solutions (10 to 100 mg/L) or dilute solutions (0.1 to 10 mg/L) by appropriate selection of titrant concentration and sample size.
b. Interferences: At pH values above 4, significant iodate formation is possible if iodine is formed in the absence of iodide;2 this results in a negative bias in titrating the first and second samples. Acidification of these samples causes reduction of iodate to iodine and a positive bias. To prevent formation of iodate add 1 g KI granules to stirred sample.
A positive bias results from oxidation of iodide to iodine by dissolved oxygen in strongly acidic solutions.1 To minimize this bias, use bromide as the reducing agent in titrating the third sample (bromide is not oxidized by oxygen under these conditions). After reaction is completed, add iodide, which will be oxidized to iodine by the bromine formed from the reduction of the original chlorine species. Add iodide carefully so that bromine gas is not lost. Rapid dilution of the sample with sodium phosphate decreases sample acidity and minimizes oxidation of iodide by oxygen. The pH of the solution to be titrated should be between 1.0 and 2.0 Carry a blank through the procedure as a check on iodide oxidation.
The potential for interferences from manganese, copper, and nitrate are minimized by buffering the sample to pH ≥4.3.4 For the method presented here, the low pH required for the chlorite and chlorate analyses provides conditions favorable to manganese, copper, and nitrite interferences.
a. Titrators: See Section 4500-Cl.D.2a through d. Amperometric titrators with a platinum-platinum electrode system are more stable and require less maintenance. (NOTE: Chlorine dioxide may attack adhesives used to connect the platinum plate to the electrode, resulting in poor readings.)
If a potentiometric titrator is used, provide a platinum sensing electrode and a silver chloride reference electrode for end-point detection.
b. Glassware: Store glassware used in this method separately from other laboratory glassware and do not use for other purposes because ClO2 reacts with glass to form a hydrophobic surface coating. To satisfy any ClO2 demand, before first use immerse all glassware in a strong ClO2 solution (200 to 500 mg/L) for 24 h and rinse only with water between uses.
c. Sampling: ClO2 is volatile and will vaporize easily from aqueous solution. When sampling a liquid stream, minimize contact with air by placing a flexible sample line to reach the bottom of the sample container, letting several container volumes overflow, slowly removing sample line, and capping container with minimum headspace. Protect from sunlight. Remove sample portions with a volumetric pipet with pipet tip placed at bottom of container. Drain pipet by placing its tip below the surface of reagent or dilution water.
a. Standard sodium thiosulfate, 0.100N: See Section 4500-Cl.B.2c.
4-57b. Standard phenylarsine oxide, 0.005 64N: See Section 4500-Cl.C.3a.
c. Phosphate buffer solution, pH 7: See Section 4500-Cl.D.3b.
d. Potassium iodide, KI, granules.
e. Saturated sodium phosphate solution: Prepare a saturated solution of Na2HPO4.12H2O with cold deionized-distilled water.
f. Potassium bromide solution, 5%: Dissolve 5 g KBr and dilute to 100 mL. Store in a brown glass-stoppered bottle. Make fresh weekly.
g. Hydrochloric acid, HCl, conc.
h. Hydrochloric acid, HCl, 2.5N: Cautiously add 200 mL conc HCl, with mixing, to distilled water, diluting to 1000 mL.
i. Purge gas: Use nitrogen gas for purging ClO2 from samples. Assure that gas is free of contaminants and pass it through a 5% KI scrub solution. Discard solution at first sign of color.
Use either sodium thiosulfate or phenylarsine oxide as titrant. Select concentration on basis of concentration range expected. The total mass of oxidant species should be no greater than about 15 mg. Make appropriate sample dilutions if necessary. A convenient volume for titration is 200 to 300 mL. Preferably analyze all samples and blanks in triplicate.
Minimize effects of pH, time, and temperature of reaction by standardizing all conditions.
a. Titration of residual chlorine and one-fifth of available ClO2: Place 1 mL pH 7 phosphate buffer in beaker and add distilled-deionized dilution water if needed. Introduce sample with minimum aeration and add 1 g KI granules while stirring. Titrate to end point (see Section 4500-Cl.D). Record reading A = mL titrant/mL sample.
b. Titration of four-fifths of available ClO2 and chlorite: Continuing with same sample, add 2 mL 2.5N HCl. Let stand in the dark for 5 min. Titrate to end point. Record reading B = mL titrant/mL sample.
c. Titration of nonvolatilized chlorine: Place 1 mL pH 7 phosphate buffer in purge vessel and add distilled-deionized dilution water if needed. Add sample and purge with nitrogen gas for 15 min. Use a gas-dispersion tube to give good gas-liquid contact. Add 1 g KI granules while stirring and titrate to end point. Record reading C = mL titrant/mL sample.
d. Titration of chlorite: Continuing with same sample, add 2 mL 2.5N HCl. Let stand in the dark for 5 min. Titrate to end point, and record reading D = mL titrant/mL sample.
e. Titration of chlorine, ClO2, chlorate, and chlorite: Add 1 mL KBr and 10 mL conc HCl to 50-mL reaction flask and mix. Carefully add 15 mL sample, with minimum aeration. Mix and stopper immediately. Let stand in the dark for 20 min. Rapidly add 1 g KI granules and shake vigorously for 5s. Rapidly transfer to titration flask containing 25 mL saturated Na2HPO4 solution. Rinse reaction flask thoroughly and add rinse water to titration flask. Final titration volume should be about 200 to 300 mL. Titrate to end point.
Repeat procedure of preceding paragraph using distilled-deionized water in place of sample to determine blank value.
Record reading E = (mL titrant sample – mL titrant blank)/mL sample.
| pH | Species | Molecular Weight mg/mol | Electrons Transferred | Equivalent Weight mg/eq |
|---|---|---|---|---|
| 7 | Chlorine dioxide | 67 452 | 1 | 67 452 |
| 2, 0.1 | Chlorine dioxide | 67 452 | 5 | 13 490 |
| 7, 2, 0.1 | Chlorine | 70 906 | 2 | 35 453 |
| 2, 0.1 | Chlorite | 67 452 | 4 | 16 863 |
| 0.1 | Chlorate | 83 451 | 6 | 13 909 |
NOTE: The 15-mL sample volume can be adjusted to provide an appropriate dilution, but maintain the ratio of sample volume can be adjusted to provide an appropriate dilution, but maintain the ratio of sample to HCl.
Because the combining power of the titrants is pH-dependent, all calculations are based on the equivalents of reducing titrant required to react with equivalents of oxidant present. Use Table 4500-ClO2:1 to obtain the equivalent weights to be used in the calculations.
In the following equations. N is the normality of the titrant used in eqivalents per liter and A through E are as defined previously.
Chlorite, mg ClO2–/L = D × N × 16 863
Chlorate, mg ClO22–/L = [E − (A + B)] × N × 13 909
Chlorine dioxide, mg ClO2/L = (5/4) × (B − D) × N × 13 490
Chlorine, mg Cl2/L = {A − [(B − D)/4]} × N × 35 453
AIETA, E.M. 1985. Amperometric analysis of chlorine dioxide, chlorine and chlorite in aqueous solution. Presented at American Water Works Assoc. Water Quality Technology Conf. 13, Houston, Texas.
GORDON, G. 1982. Improved methods of analysis for chlorate, chlorite, and hypochlorite ions. Presented at American Water Works Assoc. Water Quality Technology Conf., Nashville, Tenn.
TANG, T. F. & G. GORDON. 1980. Quantitative determination of chloride, chlorite, and chlorate ions in a mixture by successive potentiometric titrations. Anal. Chem. 52:1430.
4-58