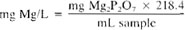
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
18TH EDITION 1992
Prepared and published jointly by:
AMERICAN PUBLIC HEALTH ASSOCIATION
AMERICAN WATER WORKS ASSOCIATION
WATER ENVIRONMENT FEDERATION
Joint Editorial Board
Arnold E. Greenberg, APHA, Chairman
Lenore S. Clesceri, WEF
Andrew D. Easton, AWWA
Managing Editor
Mary Ann H. Franson
Publication Office
American Public Health Association
1015 Fifteenth Street, NW
Washington, DC 20005
Copyright © 1917, 1920, 1923, and 1925 by
American Public Health Association
Copyright © 1933, 1936, and 1946 by
American Public Health Association
American Water Works Association
Copyright © 1955, 1960, and 1965 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1971 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1976 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1981 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1985 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1989 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1992 by
American Public Health Association
American Water Works Association
Water Environment Federation
All rights reserved. No part of this publication may be reproduced, graphically or electronically, including entering in storage or retrieval systems, without the prior written permission of the publishers.
30M7/92
The Library of Congress has catalogued this work as follows:
American Public Health Association.
Standard methods for the examination of water and wastewater.
ISBN 0-87553-207-1
Printed and bound in the United States of America.
Composition: EPS Group, Inc., Hanover, Maryland
Set in: Times Roman
Printing: Victor Graphics, Inc., Baltimore, Maryland
Binding: American Trade Bindery, Baltimore, Maryland
Cover Design: DR Pollard and Associates, Inc., Arlington, Virginia
Magnesium ranks eighth among the elements in order of abundance and is a common constituent of natural water. Important contributors to the hardness of a water, magnesium salts break down when heated, forming scale in boilers. Concentrations greater than 125 mg/L also can have a cathartic and diuretic effect. Chemical softening. reverse osmosis, electrodialysis, or ion exchange reduces the magnesium and associated hardness to acceptable levels. The magnesium concentration may vary from zero to several hundred milligrams per liter, depending on the source and treatment of the water.
The four methods presented are applicable to all natural waters. Direct determinations can be made with the atomic absorption spectrometric and inductively coupled plasma methods. Magnesium can be determined by the gravimetric method only after removal of calcium salts (see Section 3500-Ca). These methods can be applied to all concentrations by selection of suitable sample portions. Choice of method is largely a matter of personal preference and analyst experience.
* Approved by Standard Methods Committee, 1990.
3-72See flame atomic absorption spectrometric method. Section 3111B.
See Section 3120.
a. Principle: Diammonium hydrogen phosphate quantitatively precipitates magnesium in ammoniacal solution as magnesium ammonium phosphate. The precipitate is ignited to, and weighed as, magnesium pyrophosphate. A choice is presented between: (a) destruction of ammonium salts and oxalate, followed by single precipitation of magnesium ammonium phosphate; and (b) double precipitation without pretreatment. Where time is not a factor, double precipitation is preferable because, while pretreatment is faster, it requires close attention to avoid mechanical loss.
b. Interference: The solution should be reasonably free from aluminum, calcium, iron, manganese, silica, strontium, and suspended matter. It should not contain more than about 3.5 g NH4Cl.
a. Nitric acid, NHO3, conc.
b. Hydrochloric acid, HCI, conc; also 1 + 1, 1 + 9, and 1 + 99.
c. Methyl red indicator solution: Dissolve 100 mg methyl red sodium saltin distilled water and dilute to 100 mL.
d. Diammonium hydrogen phosphate solution: In distilled water, dissolve 30 g (NH4)2HPO4 and make up to 100 mL.
e. Ammonium hydroxide, NH4OH, conc; also 1 + 19.
a. By removal of oxalate and ammonium salts: To the combined filtrate and washings from the calcium determination, containing not more than 60 mg Mg, or to a portion containing less than this amount in a 600 or 800-mL beaker, add 500 mL. cone HNO3 and evaporate carefully to dryness on a hot plate. Do not let reaction become too violent during the later part of the evaporation; stay inconstant attendance to avoid losses through spattering. Moisten residue with 2 to 3 mL cone HCl; add 200 mL distilled water, warm, filter, and wash. To the filtrate add 3 mL cone HCI, 2 to 3 drops methyl red solution, and 10 mL (NH4)2HPO4 solution. Cool and add cone NH4OH, drop by drop, stirring constantly, until the color changes to yellow. Stir for 5 min, add 5 mL cone NH4OH. and stir vigorously for 10 min more. Let stand overnight and filter through filter paper.*Wash with 1 + 19 NH4OH. Transfer to an ignited, cooled, and weighted crucible Dry precipitate thoroughly and burn paper off slowly, allowing circulation of air. Heat at about 500°C until residue is white. Ignite for 30-min periods at 1100°C to constant weight.
b. By double precipitation: To the combined filtrate and washings from the calcium determination, containing not more that 60 mg Mg, or to a portion containing less than this amount, add 2 to 3 drops methyl red solution; adjust volume to 150 mL and acidify with 1 + 1 HCl. Add 10 mL (NH44)2HPO4 solution. Cool. Add cone NH4OH, drop by drop, stirring constantly, until the color changes to yellow. Stir for 5 min, add 5 mL cone NH4OH, and stir vigorously for 10 min more. Let stand overnight and then filter through filter paper.* Wash with 1 + 19 NH4OH. Discard filtrate and washings. Dissolve precipitate with 50 mL warm 1 + 9 HCl and wash paper well with hot 1 + 99 HCl. Add 2 to 3 drops methyl red solution, adjust volume to 100 to 150 mL, add 1 to 2 mL (NH4)2HPO4 solution, and precipitate as before. Let stand in a cool place for at least 4 h or preferably overnight. Filter through filter paper* and wash with 1 + 19 NH4OH. Transfer to an ignited, cooled, and weighed crucible. Dry precipicate throughly and burn paper off slowly, allowing circulation of air. Heat at about 500° until residue is white. Ignite for 30-min periods at 1100°C to constant weight.
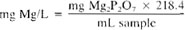
A synthetic sample containing 82 mg Mg/L, 108 mg Ca/L, 3.1 mg K/L. 19.9 mg Na/L, 241 mg Cl −/L, 1.1 mg NO3−-N/L, 0.250 mg NO2−-N/L, 259 mg SO42−/L, and 42.5 mg total alkalinity/L (contributed by NaHCO3) was analyzed in eight laboratories by the gravimetric method, with a relative standard deviation of 6.3% and a relative error of 4.9%
* Carl Schleicher and Schuell Co.. S & S No. 589 White Ribbon, or equivalent.
3-73EPPERSON, A.W. 1928. The pyrophosphate method for the determination of magnesium and phosphoric anhydride. J. Amer. Chem. Soc. 50:321.
KOLTHOFF, I.M. & E.B. SANDELL. 1952. Textbook of Quantitative Inorganic Analysis, 3rd ed. Macmillan Co., New York, N.Y., Chapter 22.
HILLEBRAND. W.F. et al. 1953. Applied Inorganic Analysis, 2nd ed. John Wiley & Sons. New York. Chapter 41 and pp. 133-134.
Magnesium may be estimated as the difference between hardness and calcium as CaCO3 if interfering metals are present in noninterfering concentrations in the calcium titration (Section 3500-Ca.D) and suitable inhibitors are used in the hardness titration (Section 2340C).
mg Mg/L = [total hardness (as mg CaCO3/L)
– calcium hardness (as mg CaCO3/L)] × 0.243
See Section 3120.
3-74