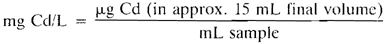
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
18TH EDITION 1992
Prepared and published jointly by:
AMERICAN PUBLIC HEALTH ASSOCIATION
AMERICAN WATER WORKS ASSOCIATION
WATER ENVIRONMENT FEDERATION
Joint Editorial Board
Arnold E. Greenberg, APHA, Chairman
Lenore S. Clesceri, WEF
Andrew D. Eaton, AWWA
Managing Editor
Mary Ann H. Franson
Publication Office
American Public Health Association
1015 Fifteenth Street, NW
Washington, DC 20005
Copyright © 1917, 1920, 1923, and 1925 by
American Public Health Association
Copyright © 1933, 1936, and 1946 by
American Public Health Association
American Water Works Association
Copyright © 1955, 1960, and 1965 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1971 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1976 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1981 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1985 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1989 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1992 by
American Public Health Association
American Water Works Association
Water Environment Federation
All rights reserved. No part of this publication may be reproduced, graphically or electronically, including entering in storage or retrieval systems, without the prior written permission of the publishers.
30M7/92
The Library of Congress has catalogued this work as follows:
American Public Health Association.
Standard methods for the examination of water and wastewater.
ISBN 0-87553-207-1
Printed and bound in the United States of America.
Composition: EPS Group, Inc., Hanover, Maryland
Set in: Times Roman
Printing: Victor Graphics, Inc., Baltimore, Maryland
Binding: American Trade Bindery, Baltimore, Maryland
Cover Design: DR Pollard and Associates, Inc., Arlington, Virginia
Cadmium is highly toxic and has been implicated in some cases of poisoning through food. Minute quantities of cadmium are suspected of being responsible for adverse changes in arteries of human kidneys. Cadmium also causes generalized cancers in laboratory animals and has been linked epidemiologically with certain human cancers. A cadmium concentration of 200 μg/L is toxic to certain fish. Cadmium may enter water as a result of industrial discharges or the deterioration of galvanized pipe.
The electrothermal (graphite furnace) atomic absorption spectrometric method is preferred. The flame atomic absorption and inductively coupled plasma methods provide acceptable precision and bias, with higher detection limits. The dithizone method is suitable when atomic absorption spectrometric or inductively coupled plasma apparatus is unavailable and the desired precision is not as great.
See flame atomic absorption spectrometric method, Sections 3111B and C, and electrothermal atomic absorption spectrometric method, Section 3113.
See Section 3120.
a. Principle: Cadmium ions under suitable conditions react with dithizone to form a pink to red color that can be extracted with chloroform (CHCl3). Chloroform extracts are measured photometrically and the cadmium concentration is obtained from a calibration curve prepared from a standard cadmium solution treated in the same manner as the sample.
b. Interference: Under the specified conditions, concentrations of metal ions normally found in water do not interfere. Lead up to 6 mg, zinc up to 3 mg, and copper up to 1 mg in the portion analyzed do not interfere. Ordinary room lighting (incandescent or fluorescent) does not affect the cadmium dithizonate color.
c. Minimum detectable concentration: 0.5 μg Cd in approximately 15 mL final volume with a 2-cm light path.
a. Colorimetric equipment: One of the following is required:
1) Spectrophotometer, for use at 518 nm with a minimum light path of 1 cm.
2) Filter photometer, equipped with a green filter having a maximum light transmittance near 518nm, with a minimum light path of 1 cm.
b. Separatory funnels, 125-mL, preferably with TFE stopcocks.
c. Glassware: Clean all glassware, including sample bottles, with 1 + 1 HCl and rinse thoroughly with tap water and distilled water.
a. Water, cadmium-free: Redistill distilled water in an all-glass still. Use this water to prepare all reagents and solutions.
b. Stock cadmium solution: Weigh 100.0 mg pure Cd metal and dissolve in a solution composed of 20 mL water plus 5 mL conc HCl. Use heat to assist metal dissolution. Transfer quantitatively to a 1-L volumetric flask and dilute to 1000 mL: 1.00 mL = 100 μg Cd. Store in polyethylene container.
c. Standard cadmium solution: Pipet 1.00 mL stock cadmium solution into a 100-mL volumetric flask, add 1 mL cone HCI, and dilute to 100 mL with water. Prepare as needed and use the same day; 1.00 mL = 1.00 μg Cd.
* Approved by Standard Methods Committee. 1990.
3-55d. Sodium potassium tartrate solution: Dissolve 250 g NaKC4H4O6·4H2O in water and make up to 1 L.
e. Sodium hydroxide-potassium cyanide solutions:
1) Solution I: Dissolve 400 g NaOH and 10 g KCN in water and make up to 1 L. Store in a polyethylene bottle. This solution is stable for 1 month.
2) Solution II: Dissolve 400 g NaOH and 0.5 g KCN in water and make up to 1 L. Store in a polyethylene bottle. This solution is stable for 1 to 2 months.
CAUTION—Potassium cyanide is extremely poisonous. Be especially cautions when handling it. Never use mouth pipets to deliver cyanide solutions.
f. Hydroxylamine hydrochloride solution: Dissolve 20 g NH2OH·HCl in water and make up to 100 mL.
g. Stock dithizone solution 1: See Section 1070D.2b1).
h. Working dithizone solution: Dilute stock dithizone solution I with CHCl3 to produce a working solution of 10 μg/mL. Prepare daily.
i. Chloroform, ACS grade passed for “suitability for use in dithizone test.” Test for a satisfactory CHCl3 by adding a minute amount of dithizone to a portion of the CHCl3 in a stoppered test tube so that a faint green is produced; the green color should be stable for a day.
j. Tartaric acid solution: Dissolve 20 g H2C4H4O6 in water and make up to 1 L. Store in refrigerator and use while still cold.
k. Hydrochloric acid, HCl, conc.
l. Thymol blue indicator solution: Dissolve 0.4 g thymolsulfonephthalein sodium salt in 100 mL water.
m. Sodium hydroxide, NaOH, 6N.
a. Preparation of standard curve: Prepare a blank and a series of standards from 1 to 10 μg by pipetting the appropriate amounts of standard Cd solution into separatory funnels. Dilute to 25 mL and proceed as in ¶ 4c. Plot a calibration curve.
b. Treatment of samples: Digest sample as directed in Section 3030. Pipet a volume of digested sample containing 1 to 10 μg Cd to a separatory funnel and dilute to 25 mL as necessary. Add 3 drops thymol blue and adjust with 6N NaOH to the first permanent yellow color, pH 2.8.
c. Color development, extraction, and measurement: Add reagents in the following order, mixing after each addition: 1 mL sodium potassium tartrate solution, 5 mL NaOH-KCN solution I, 1 mL NH2OH·HCl solution I, and 15 mL stock dithizone solution I. Stopper funnels and shake for 1 min, relieving vapor pressure in the funnels through the stopper rather than the stopcock. Drain CHCl3 layer into a second funnel containing 25 mL cold tartaric acid solution. Add 10 mL CHCl3 to first funnel; shake for 1 min and drain into second funnel. Do not permit aqueous layer to enter second funnel. Because time of contact of CHCl3 with the strong alkali must be kept to a minimum, make the two extractions immediately after adding dithizone (cadmium dithizonate decomposes on prolonged contact with strong alkali saturated with CHCl3).
Shake second funnel for 2 min and discard CHCl3 layer. Add 5 mL CHCl3, shake 1 min, and discard CHCl3 layer, making as close a separation as possible. In the following order, add 0.25 mL NH2OH·HCl solution and 15.0 mL working dithizone solution. Add 5 mL NaOH-KCN solution II; immediately shake for 1 min and transfer CHCl3 layer into a dry photometer tube. Read absorbance at 518 nm against the blank. Obtain Cd concentration from calibration curve.
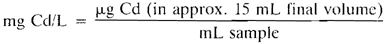
A synthetic sample containing 50 μg Cd/L, 500 μg Al/L, 110 μg Cr/L, 470 μg Cu/L, 300 μg Fe/L, 70 μg Pb/L, 150 μg Ag/L, and 650 μg Zn/L was analyzed in 44 laboratories by the dithizone method with a relative standard deviation of 24.6% and a relative error of 6.0%.
SALTZMAN, B.E. 1953. Colorimetric microdetermination of cadmium with dithizone. Anal. Chem. 25:493.
GANOTES. J., E. LARSON & R. NAVONE. 1962. Suggested dithizone method for cadmium determination. J. Amer. Water Works Assoc. 54:852.
3-56