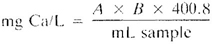
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
18TH EDITION 1992
Prepared and published jointly by:
AMERICAN PUBLIC HEALTH ASSOCIATION
AMERICAN WATER WORKS ASSOCIATION
WATER ENVIRONMENT FEDERATION
Joint Editorial Board
Arnold E. Greenberg, APHA, Chairman
Lenore S. Clesceri, WEF
Andrew D. Eaton, AWWA
Managing Editor
Mary Ann H. Franson
Publication Office
American Public Health Association
1015 Fifteenth Street, NW
Washington, DC 20005
Copyright © 1917, 1920, 1923, and 1925 by
American Public Health Association
Copyright © 1933, 1936, and 1946 by
American Public Health Association
American Water Works Association
Copyright © 1955, 1960, and 1965 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1971 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1976 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1981 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1985 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1989 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1992 by
American Public Health Association
American Water Works Association
Water Environment Federation
All rights reserved. No part of this publication may be reproduced, graphically or electronically, including entering in storage or retrieval systems, without the prior written permission of the publishers.
30M7/92
The Library of Congress has catalogued this work as follows:
American Public Health Association.
Standard methods for the examination of water and wastewater.
ISBN 0-87553-207-1
Printed and bound in the United States of America.
Composition: EPS Group, Inc., Hanover, Maryland
Set in: Times Roman
Printing: Victor Graphics, Inc., Baltimore, Maryland
Binding: American Trade Bindery, Baltimore, Maryland
Cover Design: DR Pollard and Associates, Inc., Arlington, Virginia
The presence of calcium (fifth among the elements in order of abundance) in water supplies results from passage through or over deposits of limestone, dolomite, gypsum, and gypsiferous shale. The calcium content may range from zero to several hundred milligrams per liter, depending on the source and treatment of the water. Small concentrations of calcium carbonate combat corrosion of metal pipes by laying down a protective coating. Appreciable calcium salts, on the other hand, precipitate on heating to form harmful scale in boilers, pipes, and cooking utensils. Calcium carbonate saturation in discussed in Section 2330.
* Approved by Standard Methods Committee. 1991.
3-56Calcium contributes to the total hardness of water. Chemical softening treatment, reverse osmosis, electrodialysis, or ion exchange is used to reduce calcium and the associated hardness.
The atomic absorption method and inductively coupled plasma method are accurate means of determining calcium. The EDTA titration method gives good results for control and routine applications. For samples containing high P levels (>50 mg/L) only Methods B and C are recommended because of interferences with the EDTA method using most of the cited indicators.
The customary precautions are sufficient if care is taken to redissolve any calcium carbonate that may precipitate on standing.
See flame atomic absorption spectrometric method, Section 3111B.
See Section 3120.
a. Principle: When EDTA (ethylenediaminetetraacetic acid or its salts) is added to water containing both calcium and magnesium, it combines first with the calcium. Calcium can be determined directly, with EDTA, When the pH is made sufficiently high that the magnesium is largely precipitated as the hydroxide and an indicator is used that combines with calcium only. Several indicators give a color change when all of the calcium has been complexed by the EDTA at a pH of 12 to 13.
b. Interference: Under conditions of this test, the following concentrations of ions cause no interference with the calcium hardness determination: Cu2+, 2 mg/L; Fe2+, 20 mg/L; Fe3+, 20 mg/L; Mn2+, 10 mg/L; Zn2+, 5 mg/L; Pb2+, 5 mg/L; Al3+, 5 mg/L; and Sn4+, 5 mg/L. Orthophosphate precipitates calcium at the pH of the test. Strontium and barium give a positive interference and alkalinity in excess of 300 mg/L may cause an indistinct end point in hard waters.
a. Sodium hydroxide, NaOH, 1N.
b. Indicators: Many indicators are available for the calcium titration. Some are described in the literature (see Bibliography); others are commercial preparations and also may be used. Murexide (ammonium purpurate) was the first indicator available for detecting the calcium end point, and directions for its use are presented in this procedure. Individuals who have difficulty recognizing the murexide end point may find the indicator Eriochrome Blue Black R (color index number 202) or Solochrome Dark Blue an improvement because of the color change from red to pure blue. Eriochrome Blue Black R is sodium-1-(2-hydroxy-1-naphthylazo)-2-naphthol-4-sulfonic acid. Other indicators specifically designed for use as end-point detectors in EDTA titration of calcium may be used. (Ethylenedinitrilo) tetraacetate1 indicator is especially useful in the presence of phosphate.
1) Murexide (ammonium purpurate) indicator: This indicator changes from pink to purple at the end point. Prepare by dissolving 150 mg dye in 100 g absolute ethylene glycol. Water solutions of the dye are not stable for longer than 1 d. A ground mixture of dye powder and sodium chloride (NaCl) provides a stable form of the indicator. Prepare by mixing 200 mg murexide with 100 g solid NaCl and grinding the mixture to 40 to 50 mesh. Titrate immediately after adding indicator because it is unstable under alkaline conditions. Facilitate end-point recognition by preparing a color comparison blank containing 2.0 mL NaOH solution, 0.2 g solid indicator mixture (or 1 to 2 drops if a solution is used), and sufficient standard EDTA titrant (0.05 to 0.10 mL) to produce an unchanging color.
2) Eriochrome Blue Black R indicator: Prepare a stable form of the indicator by grinding together in a mortar 200 mg powdered dye and 100 g solid NaCl to 40 to 50 mesh. Store in a tightly stoppered bottle. Use 0.2 g of ground mixture for the titration in the same manner as murexide indicator. During titration the color changes from red through purple to bluish purple to a pure blue with no trace of reddish or purple tint. The pH of some (not all) waters must be raised to 14 (rather than 12 to 13) by the use of 8N NaOH to get a good color change.
c. Standard EDTA titrant, 0.01M: Prepare standard EDTA titrant as described for the EDTA total-hardness method (Section 2340). Standard EDTA titrant, 0.0100M, is equivalent to 400.8 μg Ca/1.00 mL.
3-57a. Pretreatment of polluted water and wastewater samples: Follow the procedure described in Section 3030E or I.
b. Sample preparation: Because of the high pH used in this procedure, titrate immediately after adding alkali and indicator. Use 50.0 mL sample, or a smaller portion diluted to 50 mL so that the calcium content is about 5 to 10 mg. Analyze hard waters with alkalinity higher than 300 mg CaCO3/L by taking a smaller portion and diluting to 50 mL. or by neutralizing the alkalinity with acid, boiling 1 min, and cooling before beginning the titration.
c. Titration: Add 2.0 mL NaOH solution or a volume sufficient to produce a pH of 12 to 13. Stir. Add 0.1 to 0.2 g indicator mixture selected (or 1 to 2 drops if a solution is used). Add EDTA titrant slowly, with continuous stirring to the proper end point. When using murexide, check end point by adding 1 to 2 drops of titrant in excess to make certain that no further color change occurs.
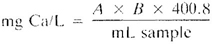
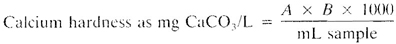
where:
A = mL titrant for sample and
B = mg CaCO3 equivalent to 1.00 mL EDTA titrant at the calcium indicator end point.
A synthetic sample containing 108 mg Ca/L, 82 mg Mg/L, 3.1 mg K/L, 19.9 mg Na/L, 241 mg Cl−/L. 1.1 mg NO3−-N/L, 0.25 mg NO2−-N/L, 259 mg SO+2−/L and 42.5 mg total alkalinity/L (contributed by NaHCO3) in distilled water was analyzed in 44 laboratories by the EDTA titrimetric method, with a relative standard deviation of 9.2% and a relative error of 1.9%.
PATTON. J. & W. REEDER 1956. New indicator for titration of calcium with (ethylenedinitrilo) tetraacetate. Anal. Chem. 28:1026.
DIEHL, H. & J. L. ELLINGBOE. 1956. Indicator for titration of calcium in the presence of magnesium using disodium dihydrogen ethylenediamine tetraacetate. Anal. Chem. 28:882.
HILDEBRAND. G. P. & C. N. REILLEY. 1957. New indicator for complexometric titration of calcium in the presence of magnesium. Anal. Chem. 29:258.
SCHWARZENBACH. G. 1957. Complexometric Titrations. Interscience Publishers, New York, N.Y.
FURMAN, N. H. 1962. Standard Methods of Chemical Analysis, 6th ed. D. Van Nostrand Co., Inc., Princeton, N. J.
KATZ. H. & R. NAVONE. 1964. Method for simultaneous determination of calcium and magnesium. J. Amer. Water Works Assoc. 56:121.
3-58