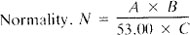
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
18TH EDITION 1992
Prepared and published jointly by:
AMERICAN PUBLIC HEALTH ASSOCIATION
AMERICAN WATER WORKS ASSOCIATION
WATER ENVIRONMENT FEDERATION
Joint Editorial Board
Arnold E. Greenberg, APHA, Chairman
Lenore S. Clesceri, WEF
Andrew D. Eaton, AWWA
Managing Editor
Mary Ann H. Franson
Publication Office
American Public Health Association
1015 Fifteenth Street, NW
Washington, DC 20005
Copyright © 1917, 1920, 1923, and 1925 by
American Public Health Association
Copyright © 1933, 1936, and 1946 by
American Public Health Association
American Water Works Association
Copyright © 1955, 1960, and 1965 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1971 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1976 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1981 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1985 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1989 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1992 by
American Public Health Association
American Water Works Association
Water Environment Federation
All rights reserved. No part of this publication may be reproduced, graphically or electronically, including entering in storage or retrieval systems, without the prior written permission of the publishers.
30M7/92
The Library of Congress has catalogued this work as follows:
American Public Health Association.
Standard methods for the examination of water and wastewater.
ISBN 0-87553-207-1
Printed and bound in the United States of America.
Composition: EPS Group, Inc., Hanover, Maryland
Set in: Times Roman
Printing: Victor Graphics, Inc., Baltimore, Maryland
Binding: American Trade Bindery, Baltimore, Maryland
Cover Design: DR Pollard and Associates, Inc., Arlington, Virginia
Alkalinity of a water is its acid-neutralizing capacity. It is the sum of all the titratable bases. The measured value may vary significantly with the end-point pH used. Alkalinity is a measure of an aggregate property of water and can be interpreted in terms of specific substances only when the chemical composition of the sample is known.
Alkalinity is significant in many uses and treatments of natural waters and wastewaters. Because the alkalinity of many surface waters is primarily a function of carbonate, bicarbonate, and hydroxide content, it is taken as an indication of the concentration of these constitutents. The measured values also may include contributions from borates phosphates, silicates, or other bases if these are present. Alkalinity in excess of alkaline earth metal concentrations is significant in determining the suitablility of a water for irrigation. Alkalinity measurements are used in the interpretation and control of water and wastewater treatment processes. Raw domestic wastewater has an alkalinity less than, or only slightly greater than, that of the water supply. Properly operating anaerobic digesters typically have supernant alkalinites in the range of 2000 to 4000 mg calcium carbonate (CaCO3)/L.1
* Approved by Standard Methods Committee. 1991.
2-25a. Principle: Hydroxyl ions present in a sample as a result of dissociation or hydrolysis of solutes react with additions of standard acid. Alkalinity thus depends on the end-point pH used. For methods of determining inflection points from titration curves and the reationale for titrating to fixed pH end points, see Section 2310B. 1a.
For samples of low alkalinity (less than 20 mg CaCO3/L) use an extrapolation technique based on the near proportionality of concentration of hydrogen ions to excess of titrant beyond the equivalence point. The amount of standard acid required to reduce pH exactly 0.30 pH unit is measured carefully. Because this change in pH corresponds to an exact doubling of the hydrogen ion concentration, a simple extrapolation can be made to the equivalence point.1,2
b. End points: When alkalinity is due entirely to carbonate or bicarbonate content, the pH at the equivalence point of the titration is determined by the concentration of carbon dioxide (CO2) at the stage. CO2 concentration depends, in turn, on the have occured during titration. The pH values in Table 2320:I are suggested as the equivalence points for the corresponding alkalinity concentrations as miligrams CaCO3 per liter. “Phenolphthalein alkalinity” is the term traditionally used for the quantity measured by titration to pH 8.3 irrespective of the colored indicator, if any, used in the determination. Phenolphthalein or metacresol purple may be used for alkalinity titration to pH 8.3. Bromcresol green or a mixed bromcresol green-methyl red indicator may be used for pH 4.5.
| Test Condition | End Point pH | |
|---|---|---|
| Total Alkalinity | Phenolphthalein Alkalinity | |
| Alkalinity | ||
| mg CaCO3/L: | ||
| 30 | 4.9 | 8.3 |
| 150 | 4.6 | 8.3 |
| 500 | 4.3 | 8.3 |
| Silicates, phosphates known or suspected | 4.5 | 8.3 |
| Routine or automated analyses | 4.5 | 8.3 |
| Industrial waste or complex system | 4.5 | 8.3 |
c. Interferences: Soaps, oily matter, suspended solids, or precipitates may coat the glass electrode and cause a sluggish response. Allow additional time between titrant additions to let electrode come to equilibrium or clean the electrodes occasionally. Do not filter, dilute, concentrate, or alter sample.
d. Selection of procedure: Determine sample alkalinity from volume of standard acid required to titrate a portion to a designated pH taken from ¶ 1b. Titrate at room temperature with a properly calibrated pH meter or electrically operated titrator, or use color indicators. If using color indicators, prepare and titrate an indicator blank.
Report alkalinity less than 20 mg CaCO3/L only if it has been determined by the low-alkalinity method of ¶ 4d.
Construct a titration curve for standardization of reagents.
Color indicators may be used for routine and control titrations in the absence of interfering color and turbidity and for preliminary titrations to select sample size and strength of titrant (see below).
e. Sample size: See Section 2310B. le for selection of size sample to be titrated and normality of titrant, substituting 0.02N or 0.1N sulfuric (H2SO4) or hydrochloric (HCl) acid for the standard alkali of that method. For the low-alkalinity method, titrate a 200-mL sample with 0.02N H2SO4 from a 10-mL buret.
f. Sampling and storage: See Section 2310B.1f.
See Section 2310B.2.
a. Sodium carbonate solution, approximately 0.05N: Dry 3 to 5 g primary standard Na2CO3 at 250°C for 4 h and cool in a desiccator. Weigh 2.5 ± 0.2 g (to the nearest mg), transfer to a 1-L volumetric flask, fill flask to the mark with distilled water, and dissolve and mix reagent. Do not keep longer than 1 week.
b. Standard sulfuric acid. 0.1N: Prepare acid solution of approximate normality as indicated under Preparation of Desk Reagents (see inside front cover). Standardize against 40.00 mL 0.05N Na2CO3 solution, with about 60 mL 0.05 N Na2CO3 solution, with about 60 mL water, in a beaker by titrating potentiometrically to pH of about 5. Lift out electrodes, rinse into the same beaker, and boil gently for 3 to 5 min under a watch glass cover. Cool to room temperature, rinse cover glass into beaker, and finish titrating to the pH inflection point. Calculate normality:
2-26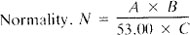
where:
A = g Na2CO3 weighed into 1-L flask.
B = mL Na2CO3 solution taken for titration, and
C = mL acid used.
Use measured normality in calculations or adjust to 0.1000N: 1 mL 0.1000N solution = 5.00 mg CaCo3.
c. Standard sulfuric acid or hydrochloric acid, 0.02N: Dilute 200.00 mL 0.1000N standard acid to 1000 mL with distilled or deionized water. Standardize by potentiometric titration of 15.00 mL 0.05N Na2CO3 according to the procedure of ¶ 3b; 1 mL = 1.00 mg CaCO3.
d. Bromcresol green indicator solution, pH 4.5 indicator: Dissolve 100 mg bromcresol green, sodium salt, in 100 mL distilled water.
e. Mixed bromcresol green-methyl red indicator solution.3 Use either the aqueous or the alcoholic solution:
1) Dissolve 100 mg bromcresol green sodium salt and 20 mg methyl red sodium salt in 100 mL distilled water.
2) Dissolve 100 mg bromcresol green and 20 mg methyl red in 100 mL 95% ethyl alcohol or isopropyl alcohol.
f. Metacresol purple indicator solution, pH 8.3 indicator: Dissolve 100 mg metacresol purple in 100 mL water.
g. Phenolphthalein solution, alcoholic, pH 8.3 indicator.
h. Sodium thiosulfate, 0.1N: See Section 2310B. 3i.
a. Color change: See Section 2310B. 4b.
b. Potentiometric titration curve: Follow the procedure for determining acidity (Section 2310B. 4c), substituting the appropriate normality of standard acid solution for standard NaOH, and continue titration to pH 4.5 or lower. Do not filter, dilute, concentrate, or alter the sample.
c. Potentiometric titration to preselected pH: Determine the appropriate end-point pH according to ¶ 1b. Prepare sample and titration assembly (Section 2310B.4c). Titrate to the end-point pH without recording intermediate pH values and without undue delay. As the end point is approached make smaller additions of acid and be sure that pH equilibrium is reached before adding more titrant.
d. Potentiometric titration of low alkalinity: For alkalinities less than 20 mg/L titrate 100 to 200 mL according to the procedure of ¶ 4c, above, using a 10-mL microburet and 0.02N standard acid solution. Stop the titration at a pH in the range 4.3 to 4.7 and record volume and exact pH. Carefully add additional titrant to reduce the pH exactly 0.30 pH unit and again record volume.
a. Potentiometric titration to end-point pH:
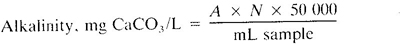
where:
A = mL standard acid used and
N = normality of standard acid
or
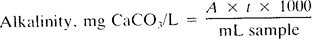
where:
i = titer of standard acid, mg CaCO3/mL.
Report pH of end point used as follows: “The alkalinity to pH______ = ______ mg CaCO3/L” and indicate clearly if this pH corresponds to an inflection point of the titration curve.
b. Potentiometric titration of low alkalinity:
Total alkalinity, mg CaCO3/L
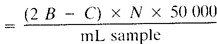
where:
B = mL titrant to first recorded pH.
C = total mL titrant to reach pH 0.3 unit lower, and
N = normality of acid.
c. Calculation of alkalinity relationships: The results obtained from the phenolphthalein and total alkalinity determinations offer a means for stoichiometric classification of the three principal forms of alkalinity present in many waters. The classification ascribes the entire alkalinity to bicarbonate, carbonate, and hydroxide, and assumes the absence of other (weak) inorganic or organic acids, such as silicic, phosphoric, and boric acids. It further presupposes the incompatibility of hydroxide and bicarbonate alkalinities. Because the calculations are made on a stoichiometric basis, ion concentrations in the strictest sense are not represented in the results, which may differ significantly from actual concentrations especially at pH > 10. According to this scheme:
1) Carbonate (CO32−) alkalinity is present when phenolphthalein alkalinity is not zero but is less than total alkalinity.
2) Hydroxide (OH−) alkalinity is present if phenolphthalein alkalinity is more than half the total alkalinity.
3) Bicarbonate (HCO3−) alkalinity is present if phenolphthalein alkalinity is less than half the total alkalinity. These relationships may be calculated by the following scheme, where P is phenolphthalein alkalinity and T is total alkalinity (¶ 1b):
Select the smaller value of P or (T−P). Then, carbonate alkalinity equals twice the smaller value. When the smaller value is P, the balance (T−2P) is bicarbonate. When the smaller value is (T−P), the balance (2P−T) is hydroxide. All results are expressed as CaCO3. The mathematical conversion of the results is shown in Table 2320:II. (A modification of Table 2320: II that is more accurate when P ≃ ½ T has been proposed.4)
Alkalinity relationships also may be computed nomographically (see Carbon Dioxide, Section 4500-CO2). Accurately measure pH, calculate OH− concentration as milligrams CaCO3 per liter, and calculate concentrations of CO32− and HCO3− as milligrams CaCO3per liter from the OH− concentration, and the phenolphthalein and total alkalinities by the following equations:
2-27CO32 = 2P − 2[OH−]
HCO3− = P − 2P +[OH−]
Similarly, if difficulty is experienced with the phenolphthalein end point, or if a check on the phenolphthalein titration is desired, calculate phenolphthalein alkalinity as CaCO3 from the results of the nomographic determinations of carbonate and hydroxide ion concentrations:
P = 1/2 [CO32−] + [OH−]
No general statement can be made about precision because of the great variation in sample characteristics. The precision of the titration is likely to be much greater than the uncertainties involved in sampling and sample handling before the analysis.
In the range of 10 to 500 mg/L, when the alkalinity is due entirely to carbonates or bicarbonates, a standard deviation of 1 mg CaCO3/L can be achieved. Forty analysts in 17 laboratories analyzed synthetic samples containing increments of bicarbonate equivalent to 120 mg CaCO3/L. The titration procedure of ¶ 4b
| Result of Titration | Hydroxide Alkalinity as CaCO3 | Carbonate Alkalinity as CaCO3 | Bicarbonate Concentration as CaCO3 |
|---|---|---|---|
| P = 0 | 0 | 0 | T |
| P < ½T | 0 | 2P | T − 2p |
| P = ½T | 0 | 2P | 0 |
| P > ½T | 2P − T | 2(T − P) | 0 |
| P = T | T | 0 | 0 |
| * Key: P − phenolphthalein alkalinity; T − Total alkalinity. | |||
was used, with an end point pH of 4.5. The standard deviation was 5 mg/L and the average bias (lower than the true value) was 9 mg/L.5
Sodium carbonates solutions equivalent to 80 and 65 mg CaCO3/L were analyzed by 12 laboratories according to the procedure of ¶ 4c.6The standard deviations were 8 and 5 mg/L, respectively, with negligible bias.6 Four laboratories analyzed six samples having total alkalinities of about 1000 mg CaCO3/L and containing various ratios of carbonate/bicarbonate by the procedures of both ¶ 4a and ¶ 4c. The pooled standard deviation was 40 mg/L, with negligible difference between the procedures.
AMERICAN SOCIETY FOR TESTING AND MATERIALS. 1982. Standard Methods for Acidity or Alkalinity of Water. Publ. D 1067-70 (reapproved 1977). American Soc. Testing & Materials. Philadelphia. Pa.
SKOUGSTAD M.W., M.J. FISHMAN, D.E. ERDMAN, & S.S. DUNCAN. 1979. Methods for determination of inorganic substances in water and fluvial sediments. In Techniques of Water-Resources Investigation of the United States Geological Survey. U.S. Geological Survey, Book 5, Chapter A1, Washington, D.C.
2-28