In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
18TH EDITION 1992
Prepared and published jointly by:
AMERICAN PUBLIC HEALTH ASSOCIATION
AMERICAN WATER WORKS ASSOCIATION
WATER ENVIRONMENT FEDERATION
Joint Editorial Board
Arnold E. Greenberg, APHA, Chairman
Lenore S. Clesceri, WEF
Andrew D. Eaton, AWWA
Managing Editor
Mary Ann H. Franson
Publication Office
American Public Health Association
1015 Fifteenth Street, NW
Washington, DC 2005
Copyright © 1917, 1920, 1923, and 1925 by
American Public Health Association
Copyright © 1933, 1936, and 1946 by
American Public Health Association
American Water Works Association
Copyright © 1955, 1960, and 1965 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1971 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1976 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1981 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1985 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1989 by
American Public Health Association
American Water Works Association
Water Pollution Control Federation
Copyright © 1992 by
American Public Health Association
American Water Works Association
Water Environment Federation
All rights reserved. No part of this publication may be reproduced, graphically or electronically, including entering in storage or retrieval systems, without the prior written permission of the publishers.
30M7/92
The Library of Congress has catalogued this work as follows:
American Public Health Association.
Standard methods for the examination of water and wastewater.
ISBN 0-87553-207-1
Printed and bound in the United States of America.
Composition: EPS Group, Inc., Hanover, Maryland
Set in: Times Roman
Printing: Victor Graphics, Inc., Baltimore, Maryland
Binding: American Trade Bindery, Baltimore, Maryland
Cover Design: DR Pollard and Associates, Inc., Arlington, Virginia
Clarity of water is important in producing products destined for human consumption and in many manufacturing uses. Beverage producers, food processors, and treatment plants drawing on a surface water supply commonly rely on coagulation, settling, and filtration to insure an acceptable product. The clarity of a natural body of water is a major determinant of the condition and productivity of that system.
Turbidity in water is caused by suspended matter, such as clay, silt, finely divided organic and inorganic matter, soluble colored organic compounds, and plankton and other microscopic organisms. Turbidity is an expression of the optical property that causes light to be scattered and absorbed rather than transmitted in straight lines through the sample. Correlation of turbidity with the weight concentration of suspended matter is difficult because the size, shape, and refractive index of the particulates also affect the light-scattering properties of the suspension. Optically black particles, such as those of activated carbon, may absorb light and effectively increase turbidity measurements.
Historically, the standard method for determination of turbidity has been based on the Jackson candle turbidimeter1; however, the lowest turbidity value that can be measured directly on this instrument is 25 units. Because turbidities of treated water usually fall within the range of 0 to 1 unit, indirect secondary methods also were developed to estimate turbidity. Unfortunately, no instrument could duplicate the results obtained on the Jackson candle turbidimeter for all samples. Because of fundamental differences in optical systems, the results obtained with different types of secondary instruments frequently do not check closely with one another, even though the instruments are precalibrated against the candle turbidimeter.
Most commercial turbidimeters available for measuring low turbidities give comparatively good indications of the intensity of light scattered in one particular direction, predominantly at right angles to the incident light. These nephelometers are unaffected relatively by small changes in design parameters and therefore are specified as the standard instrument for measurement of low turbidities. Nonstandard turbidimeters, such as forward-scattering devices, are more sensitive than nephelometers to the presence of larger particles and are useful for process monitoring.
A further cause of discrepancies in turbidity analysis is the use of suspensions of different types of particulate matter for the preparation of instrumental calibration curves. Like water samples, prepared suspensions have different optical properties depending on the particle size distributions, shapes, and refractive indices. A standard reference suspension having reproducible light-scattering properties is specified for nephelometer calibration.
Because there is no direct relationship between the intensity of light scattered at a 90° angle and Jackson candle turbidity, there is no valid basis for the practice of calibrating a nephelometer in terms of candle units. To avoid misinterpretation, report the results from nephelometric measurements as nephelometric turbidity units (NTU).
* Approved by Standard Methods Committee, 1988.
2-8Its precision, sensitivity, and applicability over a wide turbidity range make the nephelometric method preferable to visual methods. The Jackson candle method has been eliminated from the 17th and subsequent editions of Standard Methods.
Determine turbidity on the day the sample is taken. If longer storage is unavoidable, store samples in the dark for up to 24 h. Do not store for long periods because irreversible changes in turbidity may occur. Vigorously shake all samples before examination.
a. Principle: This method is based on a comparison of the intensity of light scattered by the sample under defined conditions with the intensity of light scattered by a standard reference suspension under the same conditions. The higher the intensity of scattered light, the higher the turbidity. Formazin polymer is used as the reference turbidity standard suspension. It is easy to prepare and is more reproducible in its light-scattering properties than clay or turbid natural water. The turbidity of a specified concentration of formazin suspension is defined as 40 nephelometric units. This suspension has an approximate turbidity of 40 Jackson units when measured on the candle turbidimeter; therefore, nephelometric turbidity units based on the formazin preparation will approximate units derived from the candle turbidimeter but will not be identical to them.
b. Interference: Turbidity can be determined for any water sample that is free of debris and rapidly settling coarse sediments. Dirty glassware, the presence of air bubbles, and the effects of vibrations that disturb the surface visibility of the sample will give false results. “True color,” that is, water color due to dissolved substances that absorb light, causes measured turbidities to be low. This effect usually is not significant in the case of treated water.
a. Turbidimeter consisting of a nephelometer with a light source for illuminating the sample and one or more photoelectric detectors with a readout device to indicate intensity of light scattered at 90° to the path of incident light. Use a turbidimeter designed so that little stray light reaches the detector in the absence of turbidity and free from significant drift after a short warmup period. The sensitivity of the instrument should permit detecting turbidity differences of 0.02 NTU or less in waters having turbidity of less than 1 NTU with a range from 0 to 40 NTU. Several ranges are necessary to obtain both adequate coverage and sufficient sensitivity for low turbidities.
Differences in turbidimeter design will cause differences in measured values for turbidity even though the same suspension is used for calibration. To minimize such differences, observe the following design criteria:
1) Light source—Tungsten-filament lamp operated at a color temperature between 2200 and 3000°K.
2) Distance traversed by incident light and scattered light within the sample tube—Total not to exceed 10 cm.
3) Angle of light acceptance by detector—Centered at 90° to the incident light path and not to exceed ±30° from 90°. The detector, and filter system if used, shall have a spectral peak response between 400 and 600 nm.
b. Sample tubes, clear colorless glass. Keep tubes scrupulously clean, both inside and out, and discard when they become scratched or etched. Never handle them where the light strikes them. Use tubes with sufficient extra length, or with a protective case, so that they may be handled properly. Fill tubes with samples and standards that have been agitated thoroughly and allow sufficient time for bubbles to escape.
a. Turbidity-free water: Turbidity-free water is difficult to obtain. The following method is satisfactory for measuring turbidity as low as 0.02 NTU.
Pass distilled water through a membrane filter having precision-sized holes of 0.2 µm;* the usual membrane filter used for bacteriological examinations is not satisfactory. Rinse collecting flask at least twice with filtered water and discard the next 200 mL.
Some commercial bottled demineralized waters are nearly particle-free. These may be used when their turbidity is lower than can be achieved in the laboratory. Dilute samples to a turbidity not less than I with distilled water.
b. Stock turbidity suspension:
1) Solution I—Dissolve 1.000 g hydrazine sulfate (CAUTION: Carcinogen; avoid inhalation, ingestion, and skin contact.), (NH2)2 · H2SO4, in distilled water and dilute to 100 mL in a volumetric flask.
2) Solution II—Dissolve 10.00 g hexamethylenetetramine, (CH2)6N4, in distilled water and dilute to 100 mL in a volumetric flask.
3) In a 100-mL volumetric flask, mix 5.0 mL Solution I and 5.0 mL Solution II. Let stand 24 h at 25 ± 3°C, dilute to mark, and mix. The turbidity of this suspension is 400 NTU.
4) Prepare solutions and suspensions monthly.
* Nuclepore Corporation, 7035 Commerce Circle. Pleasanton, Calif., or equivalent.
2-9c. Standard turbidity suspension: Dilute 10.00 mL stock turbidity suspension to 100 mL with turbidity-free water. Prepare daily. The turbidity of this suspension is defined as 40 NTU.
d. Alternate standards: As an alternative to preparing and diluting formazin, use commercially available standards such as styrene divinylbenzene beads† if they are demonstrated to be equivalent to freshly prepared formazin.
e. Dilute turbidity standards: Dilute portions of standard turbidity suspension with turbidity-free water as required. Prepare daily.
a. Turbidimeter calibration: Follow the manufacturer’s operating instructions. In the absence of a precalibrated scale, prepare calibration curves for each range of the instrument. Check accuracy of any supplied calibration scales on a precalibrated instrument by using appropriate standards. Run at least one standard in each instrument range to be used. Make certain that turbidimeter gives stable readings in all sensitivity ranges used. High turbidities determined by direct measurement are likely to differ appreciably from those determined by the dilution technique, ¶ 4c.
b. Measurement of turbidities less than 40 NTU: Thoroughly shake sample. Wait until air bubbles disappear and pour sample into turbidimeter tube. When possible, pour shaken sample into turbidimeter tube and immerse it in an ultrasonic bath for 1 to 2s, causing complete bubble release. Read turbidity directly from instrument scale or from appropriate calibration curve.
c. Measurement of turbidities above 40 NTU: Dilute sample with one or more volumes of turbidity-free water until turbidity falls between 30 and 40 NTU. Compute turbidity of original sample from turbidity of diluted sample and the dilution factor. For example, if five volumes of turbidity-free water were added to one volume of sample and the diluted sample showed a turbidity of 30 NTU, then the turbidity of the original sample was 180 NTU.
d. Calibrate continuous turbidity monitors for low turbidities by determining turbidity of the water entering or leaving them, using a laboratory-model turbidimeter. When this is not possible, use an appropriate dilute turbidity standard, ¶ 3e. For turbidities above 40 NTU use undiluted stock solution.
Nephelometric turbidity units (NTU) 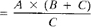
where:
A = NTU found in diluted sample,
B = volume of dilution water, mL, and
C = sample volume taken for dilution, mL.
a. Report turbidity readings as follows:
| Turbidity Range NTU | Report to the Nearest NTU |
|---|---|
| 0–1.0 | 0.05 |
| 1–10 | 0.1 |
| 10–40 | 1 |
| 40–100 | 5 |
| 100–400 | 10 |
| 400–1000 | 50 |
| >1000 | 100 |
b. For comparison of water treatment efficiencies estimate turbidity more closely than is specified above. Uncertainties and discrepancies in turbidity measurements make it unlikely that two or more laboratories will duplicate results on the same sample more closely than specified.
WHIPPLE, G.C. & D.D. JACKSON. 1900. A comparative study of the methods used for the measurement of turbidity of water. Mass. Inst. Technol. Quart. 13:274.
AMERICAN PUBLIC HEALTH ASSOCIATION. 1901. Report of Committee on Standard Methods of Water Analysis. Pub. Health Papers & Rep. 27:377.
WELLS, P.V. 1922. Turbidimetry of water, J. Amer. Water Works Assoc. 9:488.
BAYLIS, J.R. 1926. Turbidimeter for accurate measurement of low turbidities. Ind. Eng. Chem. 18:311.
WELLS, P.V. 1927. The present status of turbidity measurements. Chem. Rev. 3:331.
BAYLIS, J.R. 1933. Turbidity determinations. Water Works Sewage 80:125.
ROSE, H.E. & H.B. LLOYD. 1946. On the measurement of the size characteristics of powders by photo-extinction methods. J. Soc. Chem. Ind. (London) 65:52 (Feb.); 65:55 (Mar.).
ROSE, H.E. & C.C.J. FRENCH. 1948. On the extinction coefficient: Particle size relationship for fine mineral powders. J. Soc. Chem. Ind. (London) 67:283.
GILLETT, T.R., P.F. MEADS & A.L. HOLVEN. 1949. Measuring color and turbidity of white sugar solutions. Anal. Chem. 21:1228.
JULLANDER, I. 1949. A simple method for the measurement of turbidity. Acta Chem. Scand. 3:1309.
ROSE, H.E. 1950. Powder-size measurement by a combination of the methods of nephelometry and photo-extinction. J. Soc. Chem. Ind. (London) 69:266.
ROSE, H.E. 1950. The design and use of photoextinction sedimentometers. Engineering 169:350, 405.
BRICE, B.A., M. HALWER & R. SPEISER. 1950. Photoelectric light–scattering photometer for determining high molecular weights. J. Opt. Soc. Amer. 40:768.
KNIGHT, A.G. 1950. The measurement of turbidity in water. J. Inst. Water Eng. 4:449.
HANYA, T. 1950. Study of suspended matter in water. Bull. Chem. Soc. Jap. 23:216.
JULLANDER, I. 1950. Turbidimetric investigations on viscose. Svensk Papperstidn. 22:1.
ROSE, H.E. 1951. A reproducible standard for the calibration of turbidimeters. J. Inst. Water Eng. 5:310.
AITKEN, R.W. & D. MERCER. 1951. Comment on “The measurement of turbidity in water.” J. Inst. Water Eng. 5:328.
† AMCO-AEPA-1 Standard. Advanced Polymer Systems, 3696 C Haven Ave., Redwood City, Calif.
2-10ROSE, H.E. 1951. The analysis of water by the assessment of turbidity. J. Inst. Water Eng. 5:521.
KNIGHT, A.G. 1951. The measurement of turbidity in water: A reply. J. Inst. Water Eng. 5:633.
STAATS, F.C. 1952. Measurement of color, turbidity, hardness and silica in industrial waters. Preprint 156, American Soc. Testing & Materials, Philadelphia. Pa.
PALIN, A. T. 1955. Photometric determination of the colour and turbidity of water. Water Water Eng. 59:341.
SLOAN, C.K. 1955. Angular dependence light scattering studies of the aging of precipitates. J. Phys. Chem. 59:834.
CONLEY, W.R. & R.W. PITMAN. 1957. Microphotometer turbidity analysis. J. Amer. Water Works Assoc. 49:63.
PACKHAM, R.F. 1962. The preparation of turbidity standards. Proc. Soc. Water Treat. Exam. 11:64.
BAALSRUD, K. & A. HENRIKSEN. 1964. Measurement of suspended matter in stream water. J. Amer. Water Works Assoc. 56:1194.
HOATHER, R.C. 1964. Comparison of different methods for measurement of turbidity. Proc. Soc. Water Treat. Exam. 13:89.
EDEN, G.E. 1965. The measurement of turbidity in water. A progress report on the work of the analytical panel. Proc. Soc. Water Treat. Exam. 14:27.
BLACK, A.P. & S.A. HANNAH. 1965. Measurement of low turbidities. J. Amer. Water Works Assoc. 57:901.
HANNAH, S.A., J.M. COHEN & G.G. ROBECK, 1967, Control techniques for coagulation-filtration. J. Amer, Water Works Assoc. 59:1149.
REBHUN, M. & H.S. SPERBER. 1967. Optical properties of diluted clay suspensions. J. Colloid Interface Sci. 24:131.
DANIELS, S.L. 1969. The utility of optical parameters in evaluation of processes of flocculation and sedimentation. Chem. Eng. Progr. Symp. Ser. No. 97, 65:171.
LIVESEY, P.J. & F.W. BILLMEYER. JR. 1969. Particle-size determination by low-angle light scattering: new instrumentation and a rapid method of interpreting data. J. Colloid Interface Sci. 30:447.
OSTENDORF, R.G. & J.F. BYRD. 1969. Modern monitoring of a treated industrial effluent. J. Water Pollut. Control Fed. 41:89.
EICHNER, D. W. & C.C. HACH. 1971. How clear is clear water? Water Sewage Works 118:299.
HACH, C.C. 1972. Understanding turbidity measurement. Ind. Water Eng. 9(2):18.
SIMMS, R.J. 1972. Industrial turbidity measurement. ISA (Instrum. Soc. Amer.) Trans. 11(2):146.
TALLEY, D.G., J.A. JOHNSON & J.E. PILZER. 1972. Continuous turbidity monitoring, J. Amer. Water Works Assoc. 64:184.
2-11