
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
US EAS 76
First Edition
2006-11-14

Reference number
US EAS 76:2000
© UNBS 2006
i| Compliance with this standard does not, of itself confer immunity from legal obligations A Uganda Standard does not purport to include all necessary provisions of a contract. Users are responsible for its correct application |
| © UNBS 2006 All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilised in any form or by any means, electronic or mechanical, including photocopying and microfilm, without prior written permission from UNBS. Requests for permission to reproduce this document should be addressed to The Executive Director Uganda National Bureau of Standards P.O. Box 6329 Kampala Uganda Tel: 256 41 505 995 Fax: 256 41 286 123 E-mail: unbs@infocom.co.ug |
Uganda National Bureau of Standards (UNBS) is a parastatal under the Ministry of Tourism, Trade and Industry established, under Cap 327 of the Laws of Uganda. UNBS is mandated to co-ordinate the elaboration of standards and is
The work of preparing Uganda Standards is carried out through Technical Committees. A Technical Committee is established to deliberate on standards in a given field or area and consists of representatives of consumers, traders, academicians, manufacturers, government and other stakeholders.
Draft Uganda Standards adopted by the Technical Committee are widely circulated to stakeholders and the general public for comments. The committee reviews the comments before recommending the draft standards for approval and declaration as Uganda Standards by the National Standards Council.
This Uganda Standard, US EAS 76, Tomato products — Methods of test, is identical with and has been reproduced from an East African Standard, EAS 76:2000, Tomato products — Methods of test, and adopted as a Uganda Standard.
For the purpose of this standard, the East African Standard text should be modified as follows:
The words “this Uganda Standard” should replace the words “this East African Standard” wherever they appear.
The reference to East African Standards should be replaced by references to the appropriate Uganda Standards where they have been declared.

EAS 76:2000
ICS 67.080
EAST AFRICAN STANDARD
Tomato Products — Test Methods
EAST AFRICAN COMMUNITY
© EAC 2000
First Edition 2000
i| 1 | Scope | 1 |
| 2 | Definitions | 1 |
| 3 | Examination of containers | 1 |
| 4 | Determination of headspace and drained weight | 1 |
| 5 | General | 1 |
| 6 | Specific gravity (applicable to comminuted tomato products) | 1 |
| 7 | Total solids | 2 |
| 8 | Natural soluble solids | 3 |
| 9 | Insoluble solids | 5 |
| 10 | Benzoic acid | 8 |
| 11 | Mould count (Howard method) | 10 |
| 12 | Determination of calcium | 10 |
| 13 | Copper | 11 |
| 14 | Arsenic | 12 |
| 15 | Lead | 12 |
| 16 | Zinc | 12 |
| 17 | Determination of tin | 12 |
| 18 | Microbiological examination | 12 |
| 19 | Consistency | 12 |
| 20 | Serum colour of tomato concentrates | 4 |
| 21 | Determination of ascorbic acid | 14 |
Development of the East African Standards has been necessitated by the need for harmonizing requirements governing quality of products and services in East Africa. It is envisaged that through harmonized standardization, trade barriers which are encountered when goods and services are exchanged within the Community will be removed.
In order to achieve this objective, the Partner States in the Community through their National Bureaux of Standards, have established an East African Standards Committee.
The Committee is composed of representatives of the National Standards Bodies in Partner States, together with the representatives from the private sectors and consumer organizations. Draft East African Standards are circulated to stakeholders through the National Standards Bodies in the Partner States. The comments received are discussed and incorporated before finalization of standards, in accordance with the procedures of the Community.
East African Standards are subject to review, to keep pace with technological advances. Users of the East African Standards are therefore expected to ensure that they always have the latest versions of the standards they are implementing.
© East African Community 2000 — All rights reserved*
East African Community
P O Box 1096
Arusha
Tanzania
Tel: 255 27 2504253/8
Fax: 255-27-2504481/2504255
E-Mail: eac@eachq.org
Web: www.each.org
* © 2005 EAC — All rights of exploitation in any form and by any means reserved worldwide for EAC Partner States’ NSBs.
iii ivMethods of test for tomato products
This East African Standard specifies methods of test for tomato concentrates, modified tomato products, tomato juice and canned tomatoes.
For the purpose of this East African Standard, the following definitions shall apply.
concentrates tomato puree and tomato paste, products that are made solely by concentrating liquid obtained from substantially sound, mature red tomatoes lycopersicun esculentum P. to obtain a minimum tomato soluble solids of 8 per cent but not dehydrated to flake or powdered form.
products such as tomato sauce, tomato ketchup, or similar products which are highly seasoned products or varying concentrations containing characterizing ingredients, such as pepper, onions, vinegar, sugar, etc., in quantities that materially alter the flavour, aroma and taste of the tomato component
The condition of containers both exterior and interior shall be determined in accordance with Clause 3 of EAS 41, Methods of test for processed fruits and vegetables.
Headspace and drained weight, where necessary, shall be determined in accordance with Clauses 5 and 6 respectively of EAS 41, Methods of test for processed fruits and vegetables.
For composite solid/liquid products such as canned tomatoes, thoroughly blend the entire contents of the container in a blender. In case of comminuted products (tomato juice, tomato ketchup, purees, etc.) thoroughly shake the unopened container to incorporate any sediment, then transfer the entire contents to a large glass or porcelain dish and mix thoroughly for at least one minute. Transfer a well-mixed sample to a glass container and shake or stir thoroughly each time before removing portions for analysis.
Determine this specific gravity at 20/20 °C, using Gay Lussac or similar small neck-bottle without the cap. Clean and calibrate the bottle at 20 °C and strike off excess H2O with a straight edge, wipe the bottle dry, and weigh immediately. Cool the sample to 16 °C - 18 °C, fill the bottle with the pulp and centrifuge for 1 min at 1000 r.p.m. Add enough pulp to fill the bottle to the top and centrifuge again. Remove the bottle and take the temperature of the pulp inserting a thermometer so that no air is introduced. When the temperature is just 20 °C remove the thermometer, add enough pulp at the same temperature to have the bottle slightly over full, and strike off even with a straight edge. Clean the outside of the bottle and weigh at once to the nearest 0.01 g.
Calculate the specific gravity as follows.
1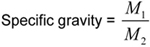
where,
M1 = mass of pulp in the bottle, and M2 = mass of H2O at 20 °C that the bottle holds.
Stainless steel, nickel or aluminium (metal) dishes, vacuum oven, desiccator.
Two methods of determination of total solids are given, the first one, (7.3) is a combination of first heating to apparent dryness and vacuum oven drying at 70 °C. (This method can be used for samples with high moisture contents which would require much longer time to dry at 70 °C in the vacuum oven.) The second method (7.4) is a straight vacuum drying method.
Weigh 15 mg diatomaceous earth filter aid to metal dishes with fitting covers (15 mg cm-2), dry for 30 min at 110 °C, cool in a desiccator, weigh. To each dish add the sample of such size that the dry residue will be between 9 mg cm-2 and 30 mg cm-2. Weigh as rapidly as possible to avoid moisture loss. Mix the sample with the filter aid and distribute uniformly over the bottom of the dish (dilute with water if necessary to facilitate distribution). Bring the sample to apparent dryness by heating on a boiling water bath or in an oven at 70 °C (with rapid air circulation). Place the partially dried sample in a vacuum oven. Admit dry air to the oven at a rate of 100 cm3 min-1 (air should be predried by passing through sulphuric acid or over activated aluminia and barium oxide). Raise the temperature to 70 ± 1 °C within the first hour of drying and lower the pressure to below 50 mm Hg. Remove dishes after 2 h, cool in a desiccator and weigh. Repeat drying for a further fifteen minutes, cool and weigh, continue to dry to constant weight. Finally cool in a desiccator (covering the sample while in a desiccator agent) and weigh as soon as possible after the sample reaches room temperature.
Calculate the total solids as follows
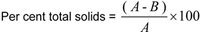
where,
A = mass of sample, and B = loss of mass on drying to constant weight.
Accurately weigh out finely ground sample into a weighed predried dish. Spread over the bottom of the dish to cover the greatest surface area and place in the vacuum Raise the oven temperature to 65 °C and lower the pressure to below 100 mm Hg, with a dry air flow of 100 cm3 min-1. Raise the dishes temperature to 70 ºC ± 1 °C within the first hour of drying and lower the pressure to below 50 mm Hg.
2Remove the dishes after 2 h, cool in a desiccator and weigh. Repeat the drying procedure for a further 15 min, cool, weigh and continue to dry to constant weight. Cool in a desiccator (as it will absorb appreciable moisture from most desiccating agents) and weigh as soon as possible after the sample reaches room temperature.
Calculate the total solids as follows
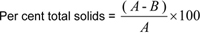
where;
A = mass of sample, B = loss of mass on drying to constant weight.
Insert folded paper, Whatman No. 2v, 12.5 cm or equivalent in a funnel of 75 cm in diameter, the stem of which has been cut off. Place the funnels in 150 ml jars of 55 mm diameter (150 ml beakers may be used, in which case the pouring spouts have to be covered with the tape to prevent evaporation).
Weigh 100 g sample at room temperature and add a weighed amount (0.2 g - 1.0 g) dry enzyme preparation. Immediately mix with a spatula to avoid evaporation and transfer to filter (8.1.1). Tanp so that the sample is in close contact with the paper, cover petri dish to have loose seal with the top of the funnel. Discard samples that do not filter within 1 h. Mix (0.2 g - 1.0 g) of dry enzyme preparation with 100 fresh sample, sealing closed containers, and incubate for 30 min-60 min at 40 °C. Cool to room temperature before opening container, remix sample and transfer to filter. For the sample that a not filter within 1 h, proceed as in (8.2.2)
With dilution (applicable to samples containing % more total solids that will not filter when treated as in (8.2.1)
Add 100 g enzyme solution to 100 and immediately mix with spatula to avoid evaporation (a mechanical mixer, e.g. Osterizer, with sealed blending container may be used). Alternately blend and shake to dislodge and break up lumps sticking to the container. Examine the mix carefully for lumps and continue mixing until homogenous transfer to the filter and cover with petri dish.
Adjust refractometer for refractive index (n) of 1.3330 with water at 20 °C. Let the sample filter into a jar or beaker until the filtrate is clear (some colour and turbid may be tolerated). Quickly remove the funnel and transfer a large drop of filtrate directly from funnel to the refractometer prism. Replace the funnel in the jar and read the refractometer preferably at 20 °C. If humidity causes condensation of moisture on prism, make the measure at room temperature according to Table 1. Read n per cent sucrose on refractometer. If n is read convert to per cent sucrose according to Table 2. Let the sample filter several minutes more. Repeat the reading by removing the funnel and transferring another drops or filtrate to the refractometer prism. The two readings should agree within 0.0002 n or 0.1 per cent sucrose. If not, repeat the readings on successive portions of the filtrate until agreement is obtained. Error readings indicate evaporation of or faulty mixing and/or filtration techniques. Read clear supernatant of 1 % solution dry enzymes on refractometer and convert to per cent sucrose.
Substract 1.15 X BC from direct reading on sample (as per cent sucrose)
where;
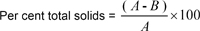
where,
1.15 = correction for insoluble solids in weighed, assuming 12.5 per cent of total solids to D = per cent enzyme preparation added to sample, and c = reading as sucrose obtained on the 1 % solution.
if diluted sample is used, subtract 0.55 X D X C from the reading (as per cent sucrose)
where,
0.55 = correction for insoluble solids as above, and D = per cent enzyme preparation added to diluting water.
Multiply corrected reading for sample 2. Add additional correction according to Table 3 to obtain per cent natural tomato solids.
To be used only when the sample contains added salt and R is greater than S. Correct refractometer reading expressed as per cent sucrose at 20 °C for added salt by the following formula:
where,
S = refractometer reading as sucrose corrected from added NaCl R = total soluble solids as sucrose
N = per cent total chlorides expressed as NaCl, determined as under 8.5 .Determination of chlorides as Nacl
Weigh 5 g material, transfer with 80 % alcohol to a 100 ml volumetric flask, and add enough 80 % alcohol to bring the volume to 50 ml. Shake well to suspend all insoluble material. Add 1 ml nitric acid with a pipette and add excess of 0.1n silver nitrate solution. Dilute to 100 ml with alcohol and transfer the mixture into a centrifuge battle and centrifuge for 5 min at 1800 r.p.m. Pipette 50 ml of the
4supernatant into 300 ml. Erlenmeyer flask, add 2 ml saturated ammonium ferrous sulphate (FeNH4 (SO4)2 solution and 2 ml nitric acid, then titrate to permanent light brown with 0.1 n ammonium cyanosulphide (NH4CNS). Divide ml of 0.1 n silver nitrate used by 2 and subtract ml of NH4CNS solution used. Multiply the difference by 0.005 844 to obtain g NaCl in sample
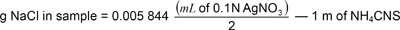
Wash 20 g of the sample repeatedly with hot water, centrifuging after each water addition and pouring out the clear supernatant through weighed filter paper on a Buchnner flask. (Filter paper used is one of the two dried for 2 h at 100 °C and weighed in a covered dish. Use a second paper, if necessary, when the first becomes clogged.) After 4 or 5 washings, transfer the remaining insoluble matter to filter, dry in a covered dish for 2 h at 100 °C, cover, cool in a desiccator, and weigh.
Per cent insoluble solids
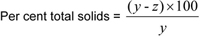
where,
y = mass of sample; and z = mass of insoluble residue
| Temperature | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 40 | 50 | 60 | 70 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtract per cent sucrose | |||||||||||
| 10 | 0.05 | 0.54 | 0.58 | 0.61 | 0.64 | 0.66 | 0.68 | 0.72 | 0.74 | 0.76 | 0.79 |
| 11 | 0.46 | 0.49 | 53 | 55 | 58 | 60 | 62 | 65 | 67 | 69 | 71 |
| 12 | 0.42 | 0.45 | 48 | 50 | 0.52 | 0.54 | 0.56 | 0.58 | 0.60 | 0.61 | 0.63 |
| 13 | 0.37 | 40 | 0.42 | 44 | 0.46 | 0.48 | 0.49 | 0.51 | 0.53 | 0.46 | 0.55 |
| 14 | 0.33 | 35 | 37 | 39 | 0.40 | 0.41 | 0.42 | 0.44 | 0.45 | 0.46 | 0.48 |
| 15 | 0.27 | 0.29 | 0.31 | 0.33 | 0.34 | 0.34 | 0.35 | 0.37 | 0.38 | 0.39 | 0.40 |
| 16 | 0.22 | 0.24 | 0.25 | 0.26 | 0.27 | 0.28 | 0.28 | 0.20 | 0.30 | 0.31 | 0.32 |
| 17 | 0.17 | 0.18 | 0.19 | 0.20 | 0.21 | 0.21 | 0.21 | 0.22 | 0.23 | 0.24 | 0.24 |
| 17 | 0.17 | 0.18 | 0.19 | 0.20 | 0.21 | 0.21 | 0.21 | 0.22 | 0.23 | 0.24 | 0.24 |
| 18 | 0.12 | 0.13 | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | 0.15 | 0.15 | 0.16 | 0.16 |
| 19 | 0.06 | 0.06 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 |
| Add | per | cent | sucrose | ||||||||
| to | 0.08 | 0.08 | 0.08 | ||||||||
| 21 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 | 0.15 | 0.15 | 0.15 | 0.08 | 0.08 | 0.08 |
| 22 | 13 | 0.13 | 0.14 | 0.14 | 0.15 | 0.23 | 0.23 | 0.24 | 0.16 | 0.16 | 0.16 |
| 23 | 19 | 0.20 | 0.21 | 0.22 | 23 | 0.30 | 0.31 | 0.31 | 0.24 | 0.24 | 0.24 |
| 24 | 26 | 0.27 | 0.28 | 0.29 | 0.30 | 0.38 | 0.39 | 0.40 | 0.31 | 0.32 | 0.32 |
| 25 | 0.33 | 0.35 | 0.36 | 0.37 | 0.38 | 0.46 | 0.47 | 0.48 | 0.40 | 0.40 | 0.40 |
| 25 | 0.33 | 0.35 | 0.36 | 0.37 | 0.38 | 0.46 | 0.47 | 0.48 | 0.40 | 0.40 | 0.40 |
| 27 | 0.48 | 0.50 | 0.52 | 0.53 | 0.54 | 0.63 | 0.63 | 0.64 | 0.56 | 0.56 | 0.56 |
| 28 | 0.56 | 0.57 | 0.60 | 0.61 | 0.62 | 0.72 | 0.72 | 0.73 | 0.64 | 0.64 | 0.65 5 |
| 29 | 0.64 | 0.66 | 0.68 | 0.69 | 0.71 | 0.80 | 0.80 | 0.81 | 0.73 | 0.73 | 0.73 |
| 30 | 0.72 | 0.74 | 0.77 | 0.78 | 0.79 | 0.81 | 0.81 | 0.81 | |||
| n | Sucrose | n | Sucrose | n | Sucrose | n | Sucrose |
|---|---|---|---|---|---|---|---|
| 20°C | per Cent | 20°C | per Cent | 20°C | per Cent | 20°C | per cent |
| values | values | values | |||||
| 1.33299 | 0.0 | 1.34027 | 5.0 | 1.34783 | 10.0 | 1.35567 | 15.0 |
| 328 | .2 | 1.34057 | .2 | 814 | .2 | 599 | .2 |
| 357 | .4 | 087 | .4 | 845 | .4 | 1.35631 | .4 |
| 385 | .6 | 116 | .6 | 875 | .6 | 664 | .6 |
| 414 | .8 | 146 | .8 | 1.34906 | .8 | 696 | .8 |
| 443 | 1.0 | 176 | 6.0 | 937 | 11.0 | 1.35728 | 16.0 |
| 472 | .2 | 206 | .2 | 968 | .2 | 760 | .2 |
| 501 | .4 | 236 | .4 | 999 | .4 | 793 | .4 |
| 530 | .6 | 266 | .6 | 1.35031 | .6 | 825 | .6 |
| 559 | .8 | 296 | .8 | 062 | .8 | 858 | .8 |
| 588 | 2.0 | 326 | 7.0 | 093 | 12.0 | 890 | 17.0 |
| 617 | .2 | 356 | .2 | 124 | .2 | 923 | .2 |
| 646 | .4 | 386 | .4 | 156 | .4 | 955 | .4 |
| 675 | .6 | 417 | .6 | 187 | .6 | 988 | .6 |
| 704 | .8 | 447 | .8 | 1.35219 | .8 | 1.35219 | .8 |
| 733 | 3.0 | 477 | 8.0 | 250 | 13.0 | 6053 | 18.0 |
| 762 | .2 | 1.34507 | .2 | 282 | .2 | 2.36086 | .2 |
| 792 | .4 | 538 | .4 | 1.35313 | .4 | 119 | .4 |
| 821 | .6 | 568 | .6 | 345 | .6 | 152 | .6 |
| 851 | .8 | 599 | .8 | 376 | .8 | 185 | .8 |
| 880 | 4.0 | 1.34629 | 9.0 | 1.35408 | 14.0 | 1.36248 | 19.0 |
| 909 | .2 | 660 | .2 | 440 | .2 | 251 | .2 |
| 939 | .4 | 691 | .4 | 472 | .4 | 248 | .4 |
| 968 | .6 | 721 | .6 | 503 | .6 | 318 | .6 |
| 998 | .8 | 752 | .8 | 535 | .8 | 351 | .8 |
| 1.36384 | 20.0 | 1.3723 | 25.0 | 1.3811 | 30.0 | 1.3902 | 35.0 |
| 417 | .2 | 26 | .2 | 15 | .2 | 06 | .2 |
| 451 | .4 | 30 | .4 | 18 | .4 | 09 | .4 |
| 484 | .6 | 33 | .6 | 22 | .6 | 13 | .6 |
| 518 | .8 | 37 | .8 | 29 | 31.0 | 20 | 36.0 |
| 585 | .2 | 44 | .2 | 25 | .8 | 16 | .8 |
| 551 | 21.0 | 40 | 26.0 | 33 | .2 | 24 | .2 |
| 618 | .4 | 47 | .4 | 36 | .4 | 28 | .4 |
| 652 | .6 | 51 | .6 | 40 | .6 | 31 | .6 |
| 685 | .8 | 54 | .8 | 43 | .8 | 35 | .8 |
| 719 | 22.0 | 58 | 27.0 | 47 | 32.0 | 39 | 37.0 |
| 753 | .2 | 61 | .2 | 51 | .2 | 43 | .2 |
| 787 | .4 | 65 | .4 | 54 | .4 | 47 | .4 |
| 820 | .6 | 68 | .6 | 58 | .6 | 50 | .6 |
| 854 | .8 | 72 | .8 | 61 | .8 | 54 | .8 |
| 888 | 23.0 | 75 | 28.0 | 65 | 33.0 | 58 | 38.0 |
| 922 | .2 | 79 | .2 | 69 | .2 | 62 | .2 |
| 956 | .4 | 82 | .4 | 72 | .4 | 66 | .4 |
| 991 | .6 | 86 | .6 | 76 | .6 | 70 | .6 |
| 1.37025 | .8 | 89 | .8 | 79 | .8 | 74 | .8 |
| 1.37059 | 24.0 | 93 | 29.0 | 83 | 34.0 | 78 | 39.0 |
| 1.3709 | .2 | 97 | .2 | 87 | .2 | 82 | .2 |
| 13 | .4 | 1.3800 | .4 | 91 | .4 | 86 | .4 |
| 16 | .6 | 04 | .6 | 94 | .6 | 89 | .6 7 |
| 20 | .8 | 07 | .8 | 98 | .8 | 93 | |
| 1.3997 | 40.0 | 1.4036 | 42.0 | 1.4076 | 44.0 | ||
| 1.4001 | .2 | 40 | .2 | 80 | .2 | ||
| 05 | .4 | 44 | .4 | 84 | .4 | ||
| 08 | .6 | 48 | .6 | 88 | .6 | ||
| 12 | .8 | 52 | .8 | 92 | .8 | ||
| 16 | 41.0 | 56 | 43.0 | 96 | 45.0 | ||
| 20 | .2 | 60 | .2 | 1.4100 | .2 | ||
| 24 | .4 | 64 | .4 | 04 | .4 | ||
| 28 | .6 | 68 | .6 | 09 | .6 | ||
| 32 | .8 | 72 | .8 | 13 | .8 |
| Natural tomato soluble solids as per cent sucrose corrected for enzyme × 2 | Correction |
|---|---|
| 25.0 | 0.3 |
| 30.0 | 0.4 |
| 35.0 | 0.5 |
| 40.0 | 0.7 |
| 45.0 | 0.8 |
| 50.0 | 0.9 |
Prepare the solution of benzoic in ether containing 50 mg/l. Determine absorbance (A) of this solution in well stoppered cuvet in a recording spectrophotometer between 265 nm and 280 nm at 1-nm intervals. Plot absorbance against wavelength and record wavelength of minimum 267.5 nm as point B, other minimum at 267.2 nm as point D, and the highest Prepare the solutions of benzoic acid ether containing 20, 40, 60, 80, 100 and 120 mg/l. Determine A of these solutions in a well stoppered cuvet in a spectrophotometer at point B, C and D. For each concentration, average A at B and D, subtract this value from A at C. Plot the difference against the concentration.
Mix the sample thoroughly, transfer 10 g or 10 ml to a separator and dilute to 200 ml with saturated NaCl solution. Make the solution definitely acid to litmus with HCl and mix well.
Extract the prepared solution with 70 ml, 50 ml, 40 ml and 30 ml portions of ether shaking well to ensure complete extraction (break emulsions by standing, stirring or centrifuging). Drain and discard aqueous phase. Wash the combined ether extracts with 50, 40 and 30 ml portions of HCl (1 volume of HCl 1 000 volumes of H2O) and discard HCl washings. If extract requires purification, proceed as follows: Extract ether solutions with 50 ml, 40 ml, 30 ml and 20 ml portions of 0.1 per cent NH4OH extracts with HCl and add 1 ml excess. Extract acidified solution with 70 ml, 50 ml, 40 mland 30 ml ether. Dilute combined ether extracts to 200 ml with ether and determine A in a well-stoppered cuvet
8in spectrophotometer at wavelength B, C and D diluting with ether if necessary to obtain optimum concentration of 20 - 120 mg/1.
Average A at B and D and subtract this value from A at C. Determine concentration of benzoic acid from the standard curve and correcting for dilutions.
Benzoic acid × 1. 18 = Na benzoate.
Conduct determination similarly on benzoate free sample of product and determine A in region 265 -280 nm at 1-nm intervals. If the curve is a straight line in this region, the method is applicable to this point.
Mix the sample thoroughly, grinding if solid or semi-solid. Transfer 150mL or 150 g to a 500-mL volumetric flask, add enough pulverized NaCl to saturated water in the sample. Make alkaline to litmus paper with 10 % NaOH solution or with milk of lime (1 part powdered recently slaked Ca (OH) 2 suspended in three parts water) and dilute to volume with saturated NaCl solution. Shake thoroughly, leave to stand for at least 2 h shaking frequently, and filter. If the sample contains large amounts of fat, portions of which may contaminate the filtrate, add a few ml of 10 % Noah solution to filtrate and extract with ether.
Add 15 g pulverized NaCl to 150 g sample, and transfer the mixture to 500 ml volumetric flask, rinsing with 150 ml saturated NaCl solution. Make slightly alkaline to litmus paper with 10 per cent NaOH solution and dilute to volume with saturated NaCl solution. Leave to stand for at least 2 h shaking frequently.
Pipette 100 ml - 200 ml of the filtrate obtained from (10.2.1.2) into a separator, neutralize to litmus paper with excess HCL (1 + 3) and add 5 ml excess. (Even where protein precipitates on acidifying, the precipitate does not interfere with extraction.) Extract carefully with chloroform (CHCl3) using successive portions of 70, 50, and 40 and 30 ml. To avoid emulsion formation, shake cautiously each time, using rotary motion. If emulsion foams, break it by stirring the CHCl3 layer with a glass rod, either by drawing off into a second separator to the other, or by extraction, carefully draw off as much clear CHCl3 solution as possible after each extracting, but do not draw off any of the emulsion with the CHCl3 layer. If this precaution is taken, CHCl3 need not be washed. Transfer combined CHCl3 extracts to porcelain evaporating dish, rinse the container several times with a few ml CHCl3 and evaporate to dryness at room temperature in a current of dry air.
Extract may also be transferred from the apparatus to 300 ml Erlenmeyer flask, and the separator rinsed with three 5 ml - 10 ml portions of CHCl3. Distil very slowly at low temperature to reduce the volume by three quarters.
Transfer the residue to porcelain evaporating dish, rinsing the flask several times with 5 ml - 10 ml portions of CHCl3 and evaporate to dryness at room temperature in a current of dry air.
Dry the residue overnight (or until no odour of acetic acid can be detected if the product is ketchup) in a desiccator containing H2SO4. Dissolve the residue of benzoic in 30 ml - 50 ml alcohol and neutralize with phenolphthalein. Add a quarter of this volume of water and 1 or 2 drops of phenolphthalein and titrate with 0.05 N NaOH.
1 ml of 0.05 N NaOH = 0.007 2 g of anhydrous sodium benzoate.
91 ml of 0.05 N NaOH = 0.007 2 g anhydrous Nabenzoate.
Alternatives 3 - 5 % pectin or 1 % algin. Add required quantity of stabilizer directly to water while agitating in high-speed blender. Treat solution with vacuum or heat to remove bubbles. Add 2 ml formaldehyde/100 ml of solution as preservative. (If blender is not available, mix dry stabilizer with alcohol to facilitate incorporation with water.) Adjust pH to 7.0 - 7.5.
In making counts for tomato products use the juice as it comes from containers. For ketchup, place some of the stabilizer solution (i) (11.1) in 100 ml granulated cylinder and 50 ml well mixed ketchup sample by displacement and mix thoroughly. In case of puree and paste, add water to make the mixture with total solids content that gives a refractive index of 1.3488 - 1.3445 at 25 °C. Clean a Howard cell so that Newton’s rings are produced between the slide and the cover glass. Remove the cover and with a scalpel place a portion of the well-mixed sample noon the central disk. Spread evenly over the disk, and cover with glass so as to give a uniform distribution. Use enough sample to bring the material to the edge of the disk. (It is of utmost importance that the portion be taken from a thoroughly mixed sample and spread evenly over the slide disk. Otherwise when the cover slip is put in place, insoluble material, and consequently moulds may be more abundant at the centre of the mount.
Discard any amount showing uneven distribution or absence of Newton’s rings or liquid that has been drawn across moat and between the cover glass and the shoulder. Place the slide under the microscope and examine with such adjustment that each field of view covers 1.5 nm2. (This area, which is essential, may frequently be obtained by so adjusting the drawtube that the diameter of the field becomes 1.382 mm. When such adjustment is not possible, make accessory drop-in ocular diaphragm with aperture accurately cut to necessary size. Diameter of the area field of view can be determined by the use of a stage micrometer. When the instrument is properly adjusted, quantity of liquid examined per field is 0.15 where identifying characteristics of mould filaments are not clearly discernible in the standard field, use magnification of 200 mm × 8 mm objective to confirm the identity of mould filaments previously observed in the least 25 fields taken in such a manner as to be representative of all sections of the mount. Observe each field, noting the presence or absence of mould filaments and recording the results as positive when the aggregate length of 3 or less filaments present exceeds one sixth of the diameter of the field. Calculate the proportion of positive fields from the results of examination of all observed fields and report as: Per cent of fields containing the mould filaments.
Distilled water or preferably deionized water;
Calcium carbonate — primary standard grade, dried at 285 °C for 2 h;
10Standard solution — 0.01 M. Dissolve 3.72 g of Na2H2EDTA.2H20 (99+ per cent purity) in water in 1 litre volumetric flask, dilute to volume and mix. Accurately weigh enough CaCO3 to give 40 ml titration with 0.01 M EDTA and transfer to 400 ml beaker. Add 50 ml of H20 and enough 10 per cent HCl to dissolve CaCO3. Dilute to 150 ml with H20 and add 15 ml of 1 N NAOH, disregarding any precipitate or turbidity. Add 200 mg of hydroxy napthol blue indicator and titrate from pink to deep blue end point, using a magnetic stirrer. Add the last few ml of EDTA solution dropwise. Molarity of EDTA solution = mg CaCO3/mL EDTA × 100.09.
KOH-KCN solution: Dissolve 280 g of KOH and 66 KCN in 1 litre of water.
Titration stand : Fluorescent illuminated. Ion exchange column :
Approximately 20mm × 600 mm, fitted with coarse porosity fritted glass disk and Teflon stopcock. Place 30 g - 40 g most Amberlite IR - 4B resin (anion exchange resin with high phosphate capacity) from a fresh bottle in a 600 ml beaker and exhaust with three 250 ml portions if 5 per cent Na2CO3 or NaOH. Wash with H20 until excess base is removed. Treat resin with three 250 portions 5 per cent HCL (3 + 22) mixing thoroughly after each treatment. Rinse the column with 250 ml H2O before each use until eluate is colourless.
Thoroughly comminute the entire contents of the can in a high-speed blender. Weigh 50 g of the sample (100 g in the absence of declaration of added calcium) into a platinum or porcelain dish. Evaporate to dryness, using a forced draft oven or other conventional means, ash at 423 °C or below 525 °C until apparently carbon-free (grey to brown). Cool, add 20 ml water, stir with a rod and add 10 ml HCL cautiously under the watch glass. Rinse off the watch glass into the dish and evaporate to dryness on a steam bath. Add 50 ml HCL (1 + 9), heat on a steam bath for 15 min and filter into 200 ml volumetric flask. Wash the paper and dish thoroughly with hot water. Cool the filtrate, dilute to the volume and mix.
Transfer 100 ml aliquot prepared sample solution to 250 ml beaker and adjust to pH 3.5 with 10 per cent KOH solution added drop wise using a pH meter and magnetic stirrer. Pass the sample solution through the resin column (column is in chloride form), collecting the effluent in 400 ml beaker at a flow rate of 2 - 3 ml/min.
Wash the column with two 50 ml portions of H2O, passing the first portion through at the same rate as the sample solution and the second at 6 - 7 min/min. Finally pass enough water freely through the column to make 250 ml - 300 ml final volume.
Adjust to pH 12.5 - 13.0, using pH meter and magnetic stirrer with KOH-KCN solution. Add 0.100 g of ascorbic acid and 200 mg - 300 mg hydroxynapthol blue indicator. Titrate immediately with 0.01 m EDTA solution through pink to deep blue end point, using a magnetic stirrer.
Per cent calcium = min EDTA × (0.01/molarity EDTA solution) × 0.400 8 × 2 × 100 mg/sample.
To be determined in accordance with EAS 41, (Fresh fruits and vegetable products— Sampling and Test methods)
11To be determined in accordance with EAS 41, (Fresh fruits and vegetable products— Sampling and Test methods)
To be determined in accordance with EAS 41, (Fresh fruits and vegetable products— Sampling and Test methods)
In accordance with EAS 41, (Fresh fruits and vegetable products— Sampling and Test methods)
Applicable to tinned tomato products, shall be determined in accordance with EAS 41, (Fresh fruits and vegetable products— Sampling and Test methods)
Shall be determined in accordance with EAS 41, (Fresh fruits and vegetable products— Sampling and Test methods)
The consistency of tomato products is affected by the amount of an extent of degradation of pectin, the sizes, shape and quantity of pulp and probably to a lesser extent the proteins, sugar and other soluble continuents. A measurement of consistency or gross viscosity is of practical importance in establishing purchasing specifications and in evaluating the overall quality of the tomato product.
A number of methods may be used for the sake of uniformity only one method will be described for a particular product and it is suggested that the details of operation be followed without deviation. The method selected is widely used and the apparatus is readily available and relatively inexpensive.
Spirit level — Must be small enough to lay in the trough of instrument.
Balance — Capacity 800 gm or more: accuracy ± 0.5 gm.
Abbe refractometer — Check adjustment with distilled water before using. Water at 25 °C should read 1.332 5.
Filtration apparatus — Whatman No. 12 filter paper or the equivalent in an appropriate holder.
Weigh out 100 gm to 300 gm paste into a tared container and add sufficient water to produce a mixture having a refractometer reading when filtered of 1.350 3 to 1.350 6 at 25 °C (13.0 ± 0.1 per cent total solids). Mix thoroughly with a spoon without incorporating air into the mixture. Check for uniformity by carefully pouring from one container to another examining for the presence, or any paste is observed sticking to the container, remix and check again by pouring from one container to another. Check temperature of the mixture and adjust to 25 °C ± 1 °C. Adjust end-to-end level of Bostwick consistometer by means of spirit level placed in the trough of the instrument. Mix the sample by stirring and immediately transfer to the sample chamber of Bostwick consistometer. Fill the chamber slightly more than level full, avoiding the incorporation of air bubbles as far as possible. Pass a straight edge across top of chamber starting from the gate end to remove excess product. Release the gate of the instrument by gradual pressure on lever holding the instrument down at the same time to prevent its movement as the gate is released.
Immediately start the stopwatch or interval timer, and after 30 s read the maximum distance of flow to the nearest 0.1 cm. Clean and dry the instrument and report the reading on another portion of the sample. (Always remix the sample before transferring to the instrument.) Do not wash the instrument with hot water if it is to be used immediately for the next determination as this may result in an increase in temperature of the sample. For highest accuracy, the instrument should be maintained at a temperature of 25 ° ± 1 °C. If readings vary by more than 0.2 cm, repeat a third time or until satisfactory agreement is obtained. Report the average of two readings or more readings, excluding any that appear to be abnormal.
NOTE The original solids of the paste may be obtained by determining the refractive index of the filtered paste and conversion of the reading to total solids. Using Tables 4 – 2 and 4 – 3. Dilution to 13 % solids may be by the following formula:
where;
Mm = mass of dilution water;
S = total solids of undiluted paste;
Mp = mass of tomato paste.
The capillary viscometer method is recommended for tomato juice. Very thick samples cannot be run by this procedure because of plugging of the capillary. Dilution with a known proportion of water might provide a means of obtaining readings on these samples.
Instrument shall be clean and dry before using. Adjust end-to-end level of Bostwick consistometer by means of spirit level placed in the trough of the instrument Adjust the sample to 20 °C ± 1 °C, mix thoroughly with spoon without incorporating air bubbles and fill the chamber slightly more than level full. Pass a straight edge across the top of chamber starting from the gate to remove excess product.
Release the gate of instrument by gradual pressure on lever holding the instrument down at the same time to prevent its movement as the gate is released. Immediately start the stopwatch or interval timer, and after 30 s read the maximum distance of the flow to the nearest 0.1 cm.
Clean and dry the instrument and repeat the reading or another portion of the sample. Do not wash the instrument with hot water if it is to be used immediately for the next determination as this may result in an increase in temperature of the sample. If readings vary by more than 0.2 cm, repeat a third time or until a satisfactory agreement is obtained. Report average of two or more readings, excluding any that appear to be abnormal.
13Serum colour is used as an index of head damage of canned tomato puree and paste and provides a measure of the expected storage life of the products. The optical density (absorbance) of filtered tomato pulp when measured at 420 mμ is found to increase as heat damage increases.
Filtration apparatus — Whatman No. 43 or the equivalent filter paper.
Reagent — Pectinal 10R or the equivalent. Make up a 5 % solution and hold in the refrigerator. Allow to settle and decant clear liquid.
Determine the refractive index of the tomato product and convert to product and convert to total solids by means of Table 4 –1 to 4 – 4. Weigh out an amount of product equal to 275 gm divided by per cent solids of the products, e.g. if product is found to contain 32.0 % solids, weigh out 275/32.0 or 8.60 gm. Transfer to a 100-mL volumetric flask, to make to volume with H2O and mix. Centrifuge for about 10 min at 2 000 r.p.m. Transfer to 40 min of centrifugate to a 150-mL stoppered Erlenmeyer flask and add 5 min of the 5 % pectinol solution. Stopper flask and set aside for 1 h to 2 h at about 40°C (104°F) or for a sufficient length of time to produce an absolutely clear filtrate when filtered. Filter through fine textured filter paper and collect filtrate in a 50-mL Erlenmeyer flask. Incubate and filter a blank containing the enzyme solution. Adjust instrument to read 100 % transmission (0 absorbance) with the ‘blank’ enzyme solution at 410 mμ and read the absorbance (optical density) of the unknown at the same wavelength. Express results as per cent absorbance. The following may be used as an approximate guide in evaluating the head damage of tomato puree and paste.
| Up to 0.10 | No heat damage; |
| 0.10 to 0.15 | Slight heat damage; |
| 0.15 to 0.20 | Moderate heat damage; |
| Over 0.20 | Severe heat damage. |
Distilled water from tin-plated copper steel often contains traces of copper, which may tender the reagents for this determination unstable and cause losses of ascorbic acid.
Traces of copper and other metallic salts may be effectively removed by passing the water through an efficient ion exchange column (‘demineralizer’) or by redistillation in a pyrex still.
Dissolve without heating 240 gm of reagent grade HPO3 sticks or pellets in about 750 min of copper-free water and dilute to 1 litre. If necessary filter to remove suspended matter. Dilute 24 % solution with copper-free water to produce a solution of 3 per cent metaphosphoric acid.
Dissolve 50 mg of sodium 2,6-dichloroindophenol and 20 mg of sodium bicarbonate in warm water and filter into a 100-mL volumetric flask using a small filter paper. Wash the filter paper until the colour is completely removed.
Pipette 25 min juice into a 100-mL volumetric flask immediately after opening tie container, and make up to volume with 3 per cent metaphosphoric acid after each time swirling or rotating the flask to bring air bubbles to the surface. If necessary add a drop of capryl alcohol to break the foam. Pipette a 10-mL aliquot of filtrate (from 5 min to 20 min may be used if desired) into a 50 min Erlenmeyer flask. Titrate rapidly with the dye solution until a definite pink colour is obtained which persists for about 15 s.
In the case of liquid products, results are preferably expressed as milligrams of ascorbic acid per 100 min the results can be calculated using the following formula:
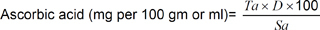
where,
15
Ta = No. of ml of titration; D = Dye factor = milligrams of ascorbic acid equivalent to 1 ml of dye; Sa = No. of min original sample titrated.
| Refractometer reading at 25 °C |
Total solids vacuum drying (AOAC method) |
Refractometer reading at 25 °C |
Total solids vacuum drying (AOAC method) |
||
|---|---|---|---|---|---|
| Refract. index |
Sugar scale |
Refract. index |
Sugar scale |
||
| 1.3376 | 3.2 | 4.0 | 1.3411 | 5.6 | 6.5 |
| 77 | 3.3 | 4.1 | 12 | 5.6 | 6.5 |
| 78 | 3.3 | 4.1 | 13 | 5.7 | 6.6 |
| 79 | 3.4 | 4.2 | 14 | 5.8 | 6.7 |
| 80 | 3.5 | 4.3 | 15 | 5.8 | 6.7 |
| 81 | 3.5 | 4.3 | 16 | 5.9 | 6.8 |
| 82 | 3.6 | 4.4 | 17 | 6.0 | 6.9 |
| 83 | 3.7 | 4.5 | 18 | 6.0 | 6.9 |
| 84 | 3.7 | 4.5 | 19 | 6.1 | 7.0 |
| 85 | 3.8 | 4.6 | 20 | 6.2 | 7.1 |
| 86 | 3.9 | 4.7 | 21 | 6.2 | 7.1 |
| 87 | 3.9 | 4.7 | 22 | 6.3 | 7.2 |
| 88 | 4.0 | 4.8 | 23 | 6.4 | 7.3 |
| 89 | 4.1 | 4.9 | 24 | 6.4 | 7.3 |
| 90 | 4.1 | 4.9 | 25 | 6.5 | 7.4 |
| 91 | 4.2 | 5.0 | 26 | 6.6 | 7.5 |
| 92 | 4.3 | 5.1 | 27 | 6.6 | 7.5 |
| 93 | 4.3 | 5.1 | 28 | 6.7 | 7.6 |
| 94 | 4.4 | 5.2 | 29 | 6.8 | 7.7 |
| 95 | 4.5 | 5.3 | 30 | 6.8 | 7.7 |
| 96 | 4.5 | 5.3 | 31 | 6.9 | 7.8 |
| 97 | 4.6 | 5.4 | 32 | 7.0 | 7.9 |
| 98 | 4.7 | 5.5 | 33 | 7.0 | 8.0 |
| 99 | 4.7 | 5.5 | 34 | 7.1 | 8.1 |
| 1.3400 | 4.8 | 5.6 | 35 | 7.2 | 8.2 |
| 1 | 4.9 | 5.7 | 36 | 7.2 | 8.2 |
| 2 | 5.0 | 5.8 | 37 | 7.3 | 8.3 |
| 3 | 5.0 | 5.9 | 38 | 7.4 | 8.4 |
| 4 | 5.1 | 6.0 | 39 | 7.4 | 8.4 |
| 5 | 5.2 | 6.1 | 40 | 7.5 | 8.5 |
| 6 | 5.2 | 6.1 | 41 | 7.6 | 8.6 |
| 7 | 5.3 | 6.2 | 42 | 7.6 | 8.6 |
| 8 | 5.4 | 6.3 | 43 | 7.7 | 8.7 |
| 9 | 5.4 | 6.3 | 44 | 7.8 | 8.8 |
| 10 | 5.5 | 6.4 | 45 | 7.8 | 8.8 |
| 1.3446 | 7.9 | 8.9 | 1.3511 | 12.1 | 13.5 |
| 47 | 8.0 | 9.0 | 12 | 12.2 | 13.6 |
| 48 | 8.0 | 9.0 | 13 | 12.2 | 13.6 |
| 49 | 8.1 | 9.1 | 14 | 12.3 | 13.7 |
| 50 | 8.2 | 9.2 | 15 | 12.4 | 13.8 |
| 51 | 8.2 | 9.2 | 16 | 12.4 | 13.8 |
| 52 | 8.3 | 9.2 | 17 | 12.5 | 13.9 |
| 53 | 8.3 | 9.4 | 18 | 12.6 | 14.0 |
| 54 | 8.4 | 9.5 | 19 | 12.6 | 14.0 |
| 55 | 8.5 | 9.6 | 20 | 12.7 | 14.1 |
| 56 | 8.5 | 9.6 | 21 | 12.7 | 14.1 |
| 57 | 8.6 | 9.7 | 22 | 12.8 | 14.2 |
| 57 | 8.7 | 9.8 | 23 | 12.9 | 14.3 |
| 59 | 8.7 | 9.8 | 24 | 12.9 | 14.3 |
| 60 | 8.8 | 9.9 | 25 | 13.0 | 14.4 |
| 61 | 8.9 | 10.0 | 26 | 13.1 | 14.5 |
| 62 | 8.9 | 10.0 | 27 | 13.1 | 14.5 |
| 63 | 9.0 | 10.1 | 28 | 13.2 | 14.6 16 |
| 64 | 9.1 | 10.2 | 29 | 13.3 | 14.7 |
| 65 | 9.1 | 10.2 | 30 | 13.3 | 14.8 |
| 66 | 9.2 | 10.3 | 31 | 13.4 | 14.9 |
| 67 | 9.3 | 10.4 | 32 | 13.4 | 14.9 |
| 68 | 9.3 | 10.4 | 33 | 13.5 | 15.0 17 |
| 69 | 9.4 | 10.5 | 34 | 13.6 | 15.1 |
| 70 | 9.5 | 10.6 | 35 | 13.6 | 15.1 |
| 71 | 9.5 | 10.6 | 36 | 13.7 | 15.2 |
| 72 | 9.6 | 10.7 | 37 | 13.8 | 15.3 |
| 73 | 9.7 | 10.8 | 38 | 13.8 | 15.3 |
| 74 | 9.7 | 10.8 | 39 | 13.9 | 15.4 |
| 75 | 9.8 | 10.9 | 40 | 13.9 | 15.4 |
| 76 | 9.9 | 11.0 | 41 | 14.0 | 15.5 |
| 77 | 9.9 | 11.1 | 42 | 14.1 | 15.6 |
| 78 | 10.0 | 11.2 | 43 | 14.1 | 15.7 |
| 79 | 10.0 | 11.2 | 44 | 14.2 | 15.8 |
| 80 | 10.1 | 11.3 | 45 | 14.3 | 15.9 |
| 81 | 10.2 | 11.4 | 11.4 | 46 | 14.3 |
| 82 | 10.2 | 11.4 | 47 | 14.4 | 16.0 |
| 83 | 10.3 | 11.5 | 48 | 14.5 | 16.4 |
| 84 | 10.4 | 11.6 | 49 | 14.5 | 16.1 |
| 85 | 10.4 | 11.6 | 50 | 14.6 | 16.2 |
| 86 | 10.5 | 11.7 | 51 | 14.6 | 16.2 |
| 87 | 10.6 | 11.8 | 52 | 14.7 | 16.3 |
| 88 | 10.6 | 11.8 | 53 | 14.8 | 16.4 |
| 89 | 10.7 | 11.9 | 54 | 14.8 | 16.4 |
| 90 | 10.8 | 12.0 | 55 | 14.9 | 16.5 |
| 91 | 10.8 | 12.1 | 56 | 15.0 | 16.6 |
| 92 | 10.9 | 12.2 | 57 | 15.0 | 16.7 |
| 93 | 11.0 | 12.3 | 58 | 15.1 | 16.8 |
| 94 | 11.0 | 12.3 | 59 | 15.1 | 16.8 |
| 95 | 11.1 | 12.4 | 60 | 15.2 | 16.9 |
| 96 | 11.1 | 12.4 | 61 | 15.3 | 17.0 |
| 97 | 11.2 | 12.4 | 62 | 15.3 | 17.0 |
| 98 | 11.3 | 12.6 | 63 | 15.4 | 17.1 |
| 99 | 11.3 | 12.6 | 64 | 15.5 | 17.2 |
| 1.3500 | 11.4 | 12.7 | 65 | 15.5 | 17.2 |
| 1 | 11.5 | 12.8 | 66 | 15.6 | 17.3 |
| 2 | 11.5 | 12.8 | 67 | 15.7 | 17.4 |
| 3 | 11.6 | 12.9 | 68 | 15.7 | 17.5 |
| 4 | 11.7 | 13.0 | 69 | 15.8 | 17.5 |
| 5 | 11.7 | 13.0 | 70 | 15.8 | 17.6 |
| 6 | 11.8 | 13.1 | 71 | 15.9 | 17.7 |
| 7 | 11.9 | 13.2 | 72 | 15.9 | 17.7 |
| 8 | 11.9 | 13.2 | 73 | 16.0 | 17.8 |
| 9 | 12.0 | 13.3 | 74 | 16.1 | 17.9 |
| 10 | 12.0 | 13.4 | 75 | 16.1 | 17.9 |
| 1.3576 | 16.2 | 18.0 | 1.3591 | 17.1 | 19.1 |
| 77 | 16.3 | 18.1 | 92 | 17.2 | 19.2 |
| 78 | 16.3 | 18.2 | 93 | 17.2 | 19.2 |
| 79 | 16.4 | 18.3 | 94 | 17.3 | 19.3 |
| 80 | 16.4 | 18.3 | 95 | 17.4 | 19.4 |
| 81 | 16.5 | 18.4 | 96 | 17.4 | 19.4 |
| 82 | 16.6 | 18.5 | 97 | 17.5 | 19.5 |
| 83 | 16.6 | 18.5 | 98 | 17.6 | 19.6 |
| 84 | 16.7 | 18.6 | 99 | 17.6 | 19.6 |
| 85 | 16.7 | 18.7 | 1.3600 | 17.7 | 19.7 |
| 86 | 16.8 | 18.8 | 1 | 17.7 | 19.8 |
| 87 | 16.9 | 18.9 | 2 | 17.8 | 19.9 18 |
| 88 | 16.9 | 18.9 | 3 | 17.9 | 20.0 |
| 89 | 17.0 | 19.0 | 4 | 17.9 | 20.0 |
| 90 | 17.0 | 19.0 | 5 | 18.0 | 20.1 |
| Refractometer reading at 25 °C |
Total solids vacuum drying (AOAC method) |
Refractometer reading at 25 °C |
Total solids vacuum drying (AOAC method) |
||
|---|---|---|---|---|---|
| Refract. index |
Sugar scale |
Refract. Index |
Sugar Scale |
||
| 1.3605 | 18.0 | 20.0 | 1.3631 | 19.6 | 21.5 |
| 6 | 18.0 | 20.0 | 32 | 19.6 | 21.5 |
| 7 | 18.1 | 20.1 | 33 | 19.7 | 21.6 |
| 8 | 18.2 | 20.2 | 34 | 19.7 | 21.6 |
| 9 | 18.2 | 20.2 | 35 | 19.8 | 21.7 |
| 10 | 18.3 | 20.3 | |||
| 11 | 18.3 | 20.3 | 36 | 19.9 | 21.8 |
| 12 | 18.4 | 20.4 | 37 | 19.9 | 21.8 |
| 13 | 18.4 | 20.5 | 38 | 20.0 | 21.9 |
| 14 | 18.5 | 20.5 | 39 | 20.0 | 21.9 |
| 15 | 18.6 | 20.6 | 40 | 20.1 | 22.0 |
| 16 | 18.6 | 20.6 | 41 | 20.2 | 22.1 |
| 17 | 18.7 | 20.7 | 42 | 20.2 | 22.1 |
| 18 | 18.8 | 20.8 | 43 | 20.3 | 22.2 |
| 19 | 18.8 | 20.8 | 44 | 20.3 | 22.2 |
| 20 | 18.9 | 20.9 | 45 | 20.4 | 22.3 |
| 21 | 19.0 | 21.0 | 46 | 20.5 | 22.4 |
| 22 | 19.0 | 21.0 | 47 | 20.5 | 22.4 |
| 23 | 19.1 | 21.1 | 48 | 20.6 | 22.5 |
| 24 | 19.1 | 21.1 | 49 | 20.6 | 22.5 |
| 25 | 19.2 | 21.2 | 50 | 20.7 | 22.6 |
| 26 | 19.3 | 21.2 | 51 | 20.8 | 22.7 |
| 27 | 19.3 | 21.2 | 52 | 20.8 | 22.7 |
| 28 | 19.4 | 21.3 | 53 | 20.9 | 22.8 |
| 29 | 19.4 | 21.3 | 54 | 20.9 | 22.8 |
| 30 | 19.5 | 21.4 | 55 | 21.0 | 22.9 |
| 1.3656 | 21.1 | 23.0 | 1.3706 | 24.0 | 25.8 |
| 57 | 21.1 | 23.0 | 7 | 24.1 | 25.9 |
| 58 | 21.2 | 23.1 | 8 | 24.1 | 25.9 |
| 59 | 21.2 | 23.1 | 9 | 24.2 | 26.0 |
| 60 | 21.3 | 23.2 | 10 | 24.2 | 26.0 |
| 61 | 21.4 | 23.3 | 11 | 24.3 | 26.1 |
| 62 | 21.4 | 23.3 | 12 | 24.4 | 26.2 |
| 63 | 21.5 | 23.4 | 13 | 24.4 | 26.2 |
| 64 | 21.5 | 23.4 | 14 | 24.5 | 26.3 |
| 65 | 21.6 | 23.5 | 15 | 24.5 | 26.3 |
| 66 | 21.6 | 23.5 | 16 | 24.6 | 26.4 |
| 67 | 21.7 | 23.5 | 17 | 24.6 | 26.4 |
| 68 | 21.8 | 23.6 | 18 | 24.7 | 26.5 |
| 69 | 21.8 | 23.6 | 19 | 24.8 | 26.6 |
| 70 | 21.9 | 23.7 | 20 | 24.8 | 26.6 |
| 71 | 21.9 | 23.7 | 21 | 24.9 | 26.7 |
| 72 | 22.0 | 23.8 | 22 | 24.9 | 26.7 |
| 73 | 22.1 | 23.9 | 23 | 25.0 | 26.8 |
| 74 | 22.1 | 23.9 | 24 | 25.1 | 26.9 |
| 75 | 22.2 | 24.0 | 25 | 25.1 | 26.9 20 |
| 76 | 22.2 | 24.0 | 26 | 25.2 | 27.0 |
| 77 | 22.3 | 24.1 | 27 | 25.2 | 27.0 |
| 78 | 22.4 | 24.2 | 28 | 25.3 | 27.1 |
| 79 | 22.4 | 24.2 | 29 | 25.4 | 27.2 |
| 80 | 22.5 | 24.3 | 30 | 25.4 | 27.2 |
| 81 | 22.5 | 24.3 | 31 | 25.5 | 27.3 |
| 82 | 22.6 | 24.4 | 32 | 25.5 | 27.3 |
| 83 | 22.7 | 24.5 | 33 | 25.6 | 27.4 |
| 84 | 22.7 | 24.5 | 34 | 25.6 | 27.4 |
| 85 | 22.8 | 24.6 | 35 | 25.7 | 27.5 |
| 86 | 22.8 | 24.6 | 36 | 25.8 | 27.6 |
| 87 | 22.9 | 24.7 | 37 | 25.8 | 27.6 |
| 88 | 23.0 | 24.8 | 38 | 25.9 | 27.7 |
| 89 | 23.0 | 24.8 | 39 | 25.9 | 27.7 |
| 90 | 23.1 | 24.9 | 40 | 26.0 | 27.8 |
| 91 | 23.1 | 24.9 | 41 | 26.1 | 27.9 |
| 92 | 23.2 | 25.0 | 42 | 26.1 | 27.9 |
| 93 | 23.2 | 25.0 | 43 | 26.2 | 28.0 |
| 94 | 23.3 | 25.1 | 44 | 26.2 | 28.0 |
| 95 | 23.4 | 25.2 | 45 | 26.3 | 28.1 |
| 96 | 23.4 | 25.2 | 46 | 26.3 | 28.1 |
| 97 | 23.5 | 25.3 | 47 | 26.4 | 28.2 |
| 98 | 23.5 | 25.3 | 48 | 26.5 | 28.3 |
| 99 | 23.6 | 25.4 | 49 | 26.5 | 28.3 |
| 1.3700 | 23.7 | 25.5 | 50 | 26.6 | 28.4 |
| 1 | 23.7 | 25.5 | 51 | 26.6 | 28.4 |
| 2 | 23.8 | 25.6 | 52 | 26.7 | 28.5 |
| 3 | 23.8 | 25.6 | 53 | 26.8 | 28.6 |
| 4 | 23.9 | 25.7 | 54 | 26.8 | 28.6 |
| 5 | 23.9 | 25.7 | 55 | 26.9 | 28.7 |
| 1.3756 | 26.9 | 28.7 | 1.3806 | 29.7 | 31.5 |
| 57 | 27.0 | 28.8 | 7 | 29.8 | 31.6 |
| 58 | 27.0 | 28.8 | 8 | 29.8 | 31.6 |
| 59 | 27.1 | 28.9 | 9 | 29.9 | 31.7 |
| 60 | 27.1 | 28.9 | 10 | 30.0 | 31.8 |
| 61 | 27.2 | 29.0 | 11 | 30.0 | 31.8 |
| 62 | 27.3 | 29.1 | 12 | 30.1 | 31.9 21 |
| 63 | 27.3 | 29.1 | 13 | 30.1 | 32.0 |
| 64 | 27.4 | 29.2 | 14 | 30.2 | 32.1 |
| 65 | 27.4 | 29.2 | 15 | 30.2 | 32.1 |
| 66 | 27.5 | 29.3 | 16 | 30.3 | 32.1 |
| 67 | 27.5 | 29.3 | 17 | 30.3 | 32.2 |
| 68 | 27.6 | 29.4 | 18 | 30.4 | 32.2 |
| 69 | 27.7 | 29.5 | 19 | 30.4 | 32.3 |
| 70 | 27.7 | 29.5 | 20 | 30.5 | 32.3 |
| 71 | 27.8 | 29.6 | 21 | 30.6 | 32.4 |
| 72 | 27.8 | 29.6 | 22 | 30.6 | 32.5 |
| 73 | 27.9 | 29.7 | 23 | 30.7 | 32.6 |
| 74 | 27.9 | 29.7 | 24 | 30.7 | 32.6 |
| 75 | 28.0 | 29.8 | 25 | 30.8 | 37.7 |
| 76 | 28.0 | 29.8 | 26 | 30.8 | 32.8 |
| 77 | 28.1 | 29.9 | 27 | 30.9 | 32.8 |
| 78 | 28.2 | 30.0 | 28 | 30.9 | 32.8 |
| 79 | 28.2 | 30.0 | 29 | 31.0 | 32.9 |
| 80 | 28.3 | 30.1 | 30 | 31.1 | 33.0 |
| 81 | 28.3 | 30.1 | 31 | 31.1 | 33.0 |
| 82 | 28.4 | 30.2 | 32 | 31.2 | 33.1 |
| 83 | 28.4 | 30.2 | 33 | 31.2 | 33.1 |
| 84 | 28.5 | 30.3 | 34 | 31.3 | 33.2 |
| 85 | 28.6 | 30.4 | 35 | 31.3 | 33.2 |
| 86 | 28.6 | 30.4 | 36 | 31.4 | 33.3 |
| 87 | 28.7 | 30.5 | 37 | 31.4 | 33.3 |
| 88 | 28.7 | 30.5 | 38 | 31.5 | 33.4 |
| 89 | 28.8 | 30.6 | 39 | 31.6 | 33.5 |
| 90 | 28.8 | 30.6 | 40 | 31.6 | 33.5 |
| 91 | 28.9 | 30.7 | 41 | 31.7 | 33.6 |
| 92 | 29.0 | 30.8 | 42 | 31.7 | 33.7 |
| 93 | 29.0 | 30.8 | 43 | 31.8 | 33.8 |
| 94 | 29.1 | 30.9 | 44 | 31.8 | 33.8 |
| 95 | 29.1 | 30.9 | 45 | 31.9 | 33.9 |
| 96 | 29.2 | 31.0 | 46 | 31.9 | 33.9 |
| 97 | 29.2 | 31.0 | 47 | 32.0 | 34.0 |
| 98 | 29.3 | 31.1 | 48 | 32.1 | 34.1 |
| 99 | 29.3 | 31.1 | 49 | 32.1 | 34.1 |
| 1.3800 | 29.4 | 31.2 | 50 | 32.2 | 34.2 |
| 1 | 29.5 | 31.3 | 51 | 32.2 | 34.2 |
| 2 | 29.5 | 31.3 | 52 | 32.3 | 34.3 |
| 3 | 29.6 | 31.4 | 53 | 32.3 | 34.3 |
| 4 | 29.6 | 31.4 | 54 | 32.4 | 34.4 |
| 5 | 29.7 | 31.5 | 55 | 32.4 | 34.4 |
| 1.3856 | 32.5 | 34.5 | 1.3861 | 32.7 | 34.7 |
| 57 | 32.5 | 34.5 | 62 | 32.8 | 34.8 |
| 58 | 32.6 | 34.6 | 63 | 32.9 | 34.9 |
| 59 | 32.6 | 34.6 | 64 | 32.9 | 34.9 |
| 60 | 32.7 | 34.7 | 65 | 33.0 | 35.0 |
| Refractometer reading at 25 °C diluted 1 +1 |
Totalsolids vacuum drying (AOAC) method) |
Refractometer reading at 25 °C diluted 1 +1 |
Total solids vacuum drying (AOAC method) |
||
|---|---|---|---|---|---|
| Refract. index |
Sugar scale |
Refract. index |
Sugar scale |
||
| 1.3458 | 8.7 | 20.0 | 1.3489 | 10.7 | 24.0 |
| 59 | 8.7 | 20.2 | 90 | 10.8 | 24.1 |
| 60 | 8.8 | 20.3 | 91 | 10.8 | 24.3 |
| 61 | 8.9 | 20.4 | 92 | 10.9 | 24.4 |
| 62 | 8.9 | 20.6 | 93 | 11.0 | 24.5 |
| 63 | 9.0 | 20.7 | 94 | 11.0 | 24.6 |
| 64 | 9.1 | 20.8 | 95 | 11.1 | 24.8 |
| 65 | 9.1 | 20.9 | 96 | 11.1 | 24.9 |
| 66 | 9.2 | 21.1 | 97 | 11.2 | 25.0 |
| 67 | 9.3 | 21.2 | 98 | 11.3 | 25.1 |
| 68 | 9.3 | 21.3 | 99 | 11.3 | 25.3 |
| 69 | 9.4 | 21.4 | 1.3500 | 11.4 | 25.4 |
| 70 | 9.5 | 21.6 | 1 | 11.5 | 25.5 |
| 71 | 9.5 | 21.7 | 2 | 11.5 | 25.7 |
| 72 | 9.6 | 21.8 | 3 | 11.6 | 25.8 |
| 73 | 9.7 | 22.0 | 4 | 11.7 | 25.9 |
| 74 | 9.7 | 22.1 | 5 | 11.7 | 26.0 |
| 75 | 9.8 | 22.2 | 6 | 11.8 | 26.2 |
| 76 | 9.9 | 22.3 | 7 | 11.9 | 26.3 |
| 77 | 9.9 | 22.5 | 8 | 11.9 | 26.4 |
| 78 | 10.0 | 22.6 | 9 | 12.0 | 26.5 |
| 79 | 10.0 | 22.7 | 10 | 12.0 | 26.7 |
| 80 | 10.1 | 22.8 | 11 | 12.1 | 26.8 |
| 81 | 10.2 | 23.0 | 12 | 12.2 | 26.9 |
| 82 | 10.2 | 23.1 | 13 | 12.2 | 27.1 |
| 83 | 10.3 | 23.2 | 14 | 12.3 | 27.2 |
| 84 | 10.4 | 23.4 | 15 | 12.4 | 27.3 |
| 85 | 10.4 | 23.5 | 16 | 12.4 | 27.4 |
| 86 | 10.5 | 23.6 | 17 | 12.5 | 27.6 |
| 87 | 10.6 | 23.7 | 18 | 12.6 | 27.7 |
| 88 | 10.6 | 23.9 | 19 | 12.6 | 27.8 |
| 20 | 12.7 | 27.9 | |||
| 1.3521 | 12.7 | 28.1 | 1.3571 | 15.9 | 34.4 |
| 22 | 12.8 | 28.2 | 72 | 15.9 | 34.6 |
| 23 | 12.9 | 28.3 | 73 | 16.0 | 34.7 |
| 24 | 12.9 | 28.5 | 74 | 16.1 | 34.8 |
| 25 | 13.0 | 28.6 | 75 | 16.1 | 35.0 |
| 26 | 13.1 | 28.7 | 76 | 16.2 | 35.1 |
| 27 | 13.1 | 28.8 | 77 | 16.3 | 35.2 |
| 28 | 13.2 | 29.0 | 78 | 16.3 | 35.3 |
| 29 | 13.3 | 29.1 | 79 | 16.4 | 35.5 |
| 30 | 13.3 | 29.2 | 80 | 16.4 | 35.6 |
| 31 | 13.4 | 29.3 | 81 | 16.5 | 35.7 |
| 32 | 13.4 | 29.5 | 82 | 16.6 | 35.8 |
| 33 | 13.5 | 29.6 | 83 | 16.6 | 36.0 |
| 34 | 13.6 | 29.7 | 84 | 16.7 | 36.1 |
| 35 | 13.6 | 29.9 | 85 | 16.8 | 36.2 |
| 36 | 13.7 | 30.0 | 86 | 16.9 | 36.3 |
| 37 | 13.8 | 30.1 | 87 | 16.9 | 36.5 |
| 38 | 13.8 | 30.2 | 88 | 17.0 | 36.6 |
| 39 | 13.9 | 30.4 | 89 | 17.0 | 36.7 |
| 40 | 13.9 | 30.5 | 90 | 17.2 | 36.8 23 |
| 41 | 14.0 | 30.6 | 91 | 17.2 | 37.0 |
| 42 | 14.1 | 30.7 | 92 | 17.3 | 37.1 |
| 43 | 14.1 | 30.9 | 93 | 17.4 | 37.2 |
| 44 | 14.2 | 31.0 | 94 | 17.4 | 37.3 |
| 45 | 14.3 | 31.1 | 95 | 17.5 | 37.5 |
| 46 | 14.3 | 31.3 | 96 | 17.6 | 37.6 24 |
| 47 | 14.4 | 31.4 | 97 | 17.6 | 37.7 |
| 48 | 14.5 | 31.5 | 98 | 17.7 | 37.8 |
| 49 | 14.5 | 31.6 | 99 | 17.7 | 38.0 |
| 50 | 14.6 | 31.8 | 1.3600 | 17.8 | 38.1 |
| 51 | 14.6 | 31.9 | 1 | 17.9 | 38.2 |
| 52 | 14.7 | 32.0 | 2 | 17.9 | 38.3 |
| 53 | 14.8 | 32.2 | 3 | 18.0 | 38.5 |
| 54 | 14.8 | 32.3 | 4 | 18.0 | 38.6 |
| 55 | 14.9 | 32.4 | 5 | 18.1 | 38.7 |
| 56 | 15.0 | 32.5 | 6 | 18.2 | 38.8 |
| 57 | 15.0 | 32.7 | 7 | 18.2 | 39.0 |
| 58 | 15.1 | 32.8 | 8 | 18.3 | 39.1 |
| 59 | 15.1 | 32.9 | 9 | 18.3 | 39.2 |
| 60 | 15.2 | 33.0 | 10 | 18.3 | 39.3 |
| 61 | 15.3 | 33.2 | 11 | 18.3 | 39.5 |
| 62 | 15.3 | 33.3 | 12 | 18.4 | 39.3 |
| 63 | 15.4 | 33.4 | 13 | 18.5 | 39.7 |
| 64 | 15.5 | 33.6 | 14 | 18.5 | 39.8 |
| 65 | 15.5 | 33.7 | 15 | 18.6 | 40.0 |
| 66 | 15.6 | 33.8 | 16 | 18.6 | 40.1 |
| 67 | 15.7 | 34.0 | 17 | 18.7 | 40.2 |
| 68 | 15.7 | 34.1 | 18 | 18.8 | 40.3 |
| 69 | 15.8 | 34.2 | 19 | 18.8 | 40.5 |
| 70 | 15.8 | 34.3 | 20 | 18.9 | 40.6 |
| 1.3621 | 19.0 | 40.7 | 1.3659 | 21.2 | 45.4 |
| 22 | 19.0 | 40.8 | 60 | 21.3 | 45.6 |
| 23 | 19.1 | 41.0 | 61 | 21.4 | 45.7 |
| 24 | 19.1 | 41.1 | 62 | 21.4 | 45.8 |
| 25 | 19.2 | 41.2 | 63 | 21.5 | 45.9 |
| 26 | 19.3 | 41.3 | 64 | 21.5 | 46.1 |
| 27 | 19.3 | 41.5 | 65 | 21.5 | 46.2 |
| 28 | 19.4 | 41.6 | 66 | 21.5 | 46.3 |
| 29 | 19.4 | 41.7 | 67 | 21.7 | 46.4 |
| 30 | 19.5 | 41.8 | 68 | 21.8 | 46.6 |
| 31 | 19.6 | 42.0 | 69 | 21.8 | 46.7 |
| 32 | 19.6 | 42.1 | 70 | 21.9 | 46.8 |
| 33 | 19.7 | 42.2 | 71 | 21.9 | 46.9 |
| 34 | 19.7 | 42.3 | 72 | 22.0 | 47.1 |
| 35 | 19.8 | 42.4 | 73 | 22.1 | 47.2 |
| 36 | 19.9 | 42.6 | 74 | 22.1 | 47.3 |
| 37 | 19.9 | 42.7 | 75 | 22.2 | 47.4 |
| 38 | 20.0 | 42.8 | 76 | 22.2 | 47.6 |
| 39 | 20.0 | 42.9 | 77 | 22.3 | 47.7 |
| 40 | 20.1 | 43.1 | 78 | 22.4 | 47.8 |
| 41 | 20.2 | 43.2 | 79 | 22.4 | 47.9 |
| 42 | 20.2 | 43.3 | 80 | 22.5 | 48.1 |
| 43 | 20.3 | 43.4 | 81 | 22.5 | 48.2 |
| 44 | 20.3 | 43.6 | 82 | 22.6 | 48.3 |
| 45 | 20.4 | 43.7 | 83 | 22.7 | 48.4 |
| 46 | 20.5 | 43.8 | 84 | 22.7 | 48.5 |
| 47 | 20.5 | 43.9 | 85 | 22.8 | 48.7 |
| 48 | 20.6 | 44.1 | 86 | 22.8 | 48.8 |
| 49 | 20.6 | 44.2 | 87 | 22.9 | 48.9 |
| 50 | 20.7 | 44.3 | 88 | 23.0 | 49.0 25 |
| 51 | 20.8 | 44.4 | 89 | 23.0 | 49.2 |
| 52 | 20.8 | 44.6 | 90 | 23.1 | 49.3 |
| 53 | 20.9 | 44.7 | 91 | 23.1 | 49.4 |
| 54 | 20.9 | 44.8 | 92 | 23.2 | 49.5 |
| 55 | 21.0 | 44.9 | 93 | 23.2 | 49.7 |
| 56 | 21.1 | 45.1 | 94 | 23.3 | 49.8 |
| 57 | 21.1 | 45.2 | 95 | 23.4 | 49.9 |
| 58 | 21.2 | 45.3 | 96 | 23.4 | 50.0 |
| Refractometer reading at 25 °C |
Total solids vacuum drying (AOAC Method) |
Refractometer reading at 25 °C |
Total solids vacuum drying (AOAC method) |
||
|---|---|---|---|---|---|
| Refract. index |
Sugar scale |
Refract. index |
Sugar scale |
||
| 1.3584 | 16.7 | 18.0 | 1.3747 | 26.4 | 27.2 |
| 1.3587 | 16.9 | 18.2 | 1.3750 | 26.6 | 27.4 |
| 1.3591 | 17.1 | 18.4 | 1.3754 | 26.8 | 27.6 |
| 1.3594 | 17.3 | 18.6 | 1.3757 | 27.0 | 27.8 |
| 1.3598 | 17.5 | 18.8 | 1.3761 | 27.2 | 28.0 |
| 1.3601 | 17.7 | 19.0 | 1.3764 | 27.4 | 28.2 |
| 1.3605 | 18.0 | 19.2 | 1.3768 | 27.6 | 28.4 |
| 1.3608 | 18.2 | 19.4 | 1.3771 | 27.8 | 28.6 |
| 1.3612 | 18.4 | 19.6 | 1.3775 | 28.0 | 28.8 |
| 1.3616 | 18.6 | 19.8 | 1.3778 | 28.2 | 29.0 |
| 1.3619 | 18.9 | 20.0 | 1.3782 | 28.4 | 29.2 |
| 1.3623 | 19.1 | 20.2 | 1.3786 | 28.6 | 29.4 |
| 1.3626 | 19.3 | 20.4 | 1.3786 | 28.6 | 29.4 |
| 1.3630 | 19.5 | 20.6 | 1.3789 | 28.8 | 29.6 |
| 1.3633 | 19.7 | 20.8 | 1.3793 | 29.0 | 29.8 |
| 1.3637 | 19.9 | 21.0 | 1.3796 | 29.2 | 30.0 |
| 1.3640 | 20.1 | 21.2 | 1.3800 | 29.4 | 30.2 |
| 1.3644 | 20.3 | 21.4 | 1.3803 | 29.6 | 30.4 |
| 1.3647 | 20.5 | 21.6 | 1.3807 | 29.8 | 30.6 |
| 1.3651 | 20.8 | 21.8 | 1.3810 | 30.0 | 30.8 |
| 1.3655 | 21.0 | 22.0 | 1.3814 | 30.2 | 31.0 |
| 1.3658 | 21.2 | 22.2 | 1.3817 | 30.4 | 31.2 |
| 1.3662 | 21.4 | 22.4 | 1.3821 | 30.6 | 31.4 |
| 1.3665 | 21.6 | 22.6 | 1.3824 | 30.8 | 31.6 |
| 1.3669 | 21.8 | 22.8 | 1.3828 | 31.0 | 31.8 |
| 1.3672 | 22.0 | 23.0 | 1.3832 | 31.2 | 32.0 |
| 1.3676 | 22.2 | 23.2 | 1.3835 | 31.4 | 32.2 |
| 1.3679 | 22.4 | 23.4 | 1.3839 | 31.6 | 32.4 |
| 1.3683 | 22.7 | 23.6 | 1.3842 | 31.7 | 32.6 |
| 1.3686 | 22.9 | 23.8 | 1.3846 | 31.9 | 32.8 |
| 1.3690 | 23.1 | 24.0 | 1.3849 | 32.1 | 33.0 |
| 1.3693 | 23.3 | 24.2 | 1.3853 | 32.3 | 33.2 |
| 1.3697 | 23.5 | 24.4 | 1.3856 | 32.5 | 33.4 |
| 1.3701 | 23.7 | 24.6 | 1.3860 | 32.7 | 33.6 |
| 1.3704 | 23.9 | 24.8 | 1.3863 | 32.9 | 33.8 |
| 1.3708 | 24.1 | 25.0 | 1.3867 | 33.1 | 34.0 |
| 1.3711 | 24.3 | 25.2 | 1.3871 | 33.1 | 34.2 |
| 1.3715 | 24.5 | 25.4 | 1.3874 | 33.5 | 34.4 |
| 1.3718 | 24.7 | 25.6 | 1.3878 | 33.7 | 34.6 |
| 1.3722 | 24.9 | 25.8 | 1.3881 | 33.9 | 34.8 |
| 1.3725 | 25.1 | 26.0 | 1.3885 | 34.1 | 35.0 |
| 1.3729 | 25.4 | 26.2 | 1.3888 | 34.3 | 35.2 |
| 1.3732 | 25.6 | 26.4 | 1.3892 | 34.5 | 35.4 |
| 1.3736 | 25.8 | 26.6 | 1.3895 | 34.6 | 35.6 |
| 1.3739 | 26.0 | 26.8 | 1.3899 | 34.8 | 35.8 |
| 1.3906 | 35.2 | 36.2 | 1.3941 | 37.1 | 38.2 |
| 1.3909 | 35.4 | 36.4 | 1.3945 | 37.3 | 38.4 |
| 1.3913 | 35.6 | 36.6 | 1.3948 | 37.5 | 38.6 |
| 1.3917 | 35.8 | 36.8 | 1.3952 | 37.7 | 38.8 |
| 1.3924 | 36.2 | 37.2 | 1.3959 | 38.0 | 39.2 |
| 1.3927 | 36.4 | 37.4 | 1.3963 | 38.2 | 39.4 |
| 1.3931 | 36.6 | 37.6 | 1.3966 | 38.4 | 39.6 |
| 1.3934 | 36.8 | 37.8 | 1.3970 | 38.6 | 39.8 27 |
| 1.3938 | 37.0 | 38.0 | 1.3973 | 38.8 | 40.0 |