
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.

EAS 187:2000
ICS 71.100.70
EAST AFRICAN COMMUNITY
© EAC 2000
First Edition 2000
iDevelopment of the East African Standards has been necessitated by the need for harmonizing requirements governing quality of products and services in East Africa. It is envisaged that through harmonized standardization, trade barriers which are encountered when goods and services are exchanged within the Community will be removed.
In order to achieve this objective, the Partner States in the Community through their National Bureaux of Standards, have established an East African Standards Committee.
The Committee is composed of representatives of the National Standards Bodies in Partner States, together with the representatives from the private sectors and consumer organizations. Draft East African Standards are circulated to stakeholders through the National Standards Bodies in the Partner States. The comments received are discussed and incorporated before finalization of standards, in accordance with the procedures of the Community.
East African Standards are subject to review, to keep pace with technological advances. Users of the East African Standards are therefore expected to ensure that they always have the latest versions of the standards they are implementing.
© East African Community 2000 — All rights reserved*
East African Community
P O Box 1096
Arusha
Tanzania
Tel: 255 27 2504253/8
Fax: 255-27-2504481/2504255
E-Mail: eac@eachq.org
Web: www.each.org
* ©2000 EAC — All rights of exploitation in any form and by any means reserved worldwide for EAC Partner States’ NSBs.
iiThe need for the development of an East African Standard on toothpaste has arisen from the fast expanding toothpaste industry within the region. Toothpaste assists in the removal of daily accumulation of debris and deposits from the exposed surface of the teeth without causing injury to the teeth and mucous membrane of the mouth. In addition it may remove stain and odour but it shall not remove enamel of the teeth.
iii| 1 | Scope | 1 |
| 2 | Definitions | 1 |
| 3 | Requirements for toothpaste | 1 |
| 4 | Prohibition or restrictions on clinical claims | 3 |
| 5 | Sampling, testing and compliance with the standard | 3 |
| 6 | Packing and marking requirements | 4 |
| Annex A (normative) Determination of lead content | 5 | |
| Annex B (normative) Determination of pH | 9 | |
| Annex C (normative) Test for stability | 10 | |
| Annex D (normative) Determination of corrosion of the container | 11 | |
| Annex E (normative) Determination of extrusion | 12 | |
| Annex F (normative) Determination of fluoride content | 13 | |
| Annex G (normative) Determination of fineness | 15 | |
| Annex H (normative) Determination of arsenic (alternative method-modified Gutzeit method | 16 | |
| Annex I (informative) Bibliography | 17 | |
Toothpaste — Specification
This East African Standard specifies the requirements for toothpaste (fluoridated and non-fluoridated) for use with a brush in the cleaning of natural teeth.
For the purpose of this standard the following definitions apply.
The calcified tissue, tubular in structure, that forms the bulk of a tooth.
The highly calcified tissue, prismatic in structure, that covers the crown of a tooth.
One unit of the final product consisting of the closed tube filled with toothpaste. The tube may be contained in a box (usually carton).
A pack containing two or more product units of the same nominal volume.
Collection of product units of the same size, type and style which have been manufactured under essentially the same conditions.
A product unit that fails to meet one or more of the requirements as set out in 4.3 and 5.2.
The toothpaste shall not contain sucrose or other readily fermentable carbohydrates. Artificial sweeteners, if used, shall comply with the relevant East African Standard
Colouring matter, if used, shall comply with the requirements of EAS 103.
All ingredients shall be of acceptable quality and shall meet the requirements of the relevant East African Standards
The toothpaste shall be smooth and free from lumps or particles, which are palpable in the mouth as separate or discrete particles.
The paste shall extrude from the tube at 27°C ± 2 °C in the form of a homogeneous mass with the application of normal force starting from one end of the tube.
1The toothpaste shall not contain ingredients in sufficient concentration to cause a toxic or irritant reaction when used in the oral cavity, nor shall it be otherwise harmful in normal use.
The lead content of the toothpaste shall not exceed 0.5 mg/kg, when tested in accordance with Annex A.
The pH value of the toothpaste shall be neither below 5.5 nor above 10.5, when tested in accordance with Annex B.
The toothpaste shall not ferment, segregate or otherwise deteriorate, during normal conditions of storage and use. When tested in accordance with annex C, the fore mentioned defects shall not occur.
Tooth paste shall be packed in suitable tubes of material which shall not corrode, deteriorate, or cause contamination of the tooth paste during normal conditions of storage and use. The paste shall be examined visually by extruding part of the contents. The internal surface of the tube when examined in accordance with Annex D shall show no visible signs of corrosion or deterioration and the toothpaste shall show no sign of contamination.
The lead content of the paints on the tube shall not exceed 250 mg/kg, when tested in accordance with Annex A.
The toothpaste shall extrude to the extent of 94 % of the nominal volume, when tested in accordance with Annex E.
The total fluorine derivative and water-soluble fluorine derivative in fluoridated toothpaste (both determined as fluoride ion) shall comply with the requirements given in Table 1. The values given in the table are considered acceptable for toothpaste formulated to contain 1000 mg/kg expressed as fluoride ion.
NOTE The therapeutic effectiveness of a fluoride toothpaste may be influenced by the combination of fluorine containing additive, abrasive agent and other constituents in toothpaste. Combinations additional to those in the table are acceptable provided that the product complies with all requirements of this standard with specific reference to clause 3.
2| Predominant abrasive agent in toothpaste | Permitted range of total fluorine derivatives as fluoride ion, mg/kg | Minimum soluble fluorine derivative as fluoride ion, mg/kg |
|---|---|---|
| Fluoride agent: Stannous fluoride Insoluble sodium metaphosphate Silica Calcium pyrophosphate |
900 to 1120 | 500 500 108 |
| Fluoride agent: Sodiumr Monofluorophosphate Alumina Calcium carbonate Dicalcium phosphate Insoluble sodium metaphosphate Silica |
) ) ) 850 to 1120 ) ) |
) ) ) ) 600 ) ) ) |
| Fluoride agent: Sodium fluoride High betaphase calcium Pyrophosphate Polymethacrylate spheres |
) ) 900 to 1150 ) ) |
403 600 |
Test methods specified in Annex F are suitable for sodium monofluorophosphate formulations. If formulations involving other fluoride agents are used, then the test methods have to be approved by the respective partner states National Standards Bodies.
The toothpaste shall also comply with requirements given in Table 2.
| S/N | Characteristic | Requirements | Method of test |
|---|---|---|---|
| 1 | Lead (Pb) mg/kg, max | 5 | Annex A |
| 2 | pH | 4.0 to 10.5 | Annex B |
| 3 | Fineness | 0.1 | Annex G |
| Particles retained on a 150 micron sieve, per cent by mass, max | |||
| Particles retained on a 75 micron sieve, per cent by mass, max | 2.0 | Annex G | |
| 4 | Arsenic (As2O3) mg/kg, max | 2 | Annex H or (EAS 120) |
The toothpaste shall not be claimed to control the amount of dental plaque, remove calculus, reduce dentine hypersensitivity, reduce the incidence of or treat caries or periodontal disease or any other oral diseases, unless such benefits have been clearly demonstrated by clinical trials or other scientific evidence that are acceptable to the respective partner states National Standards Bodies.
Select at random sixty bulk packs. From each bulk pack thus selected, draw at random, one product unit. Divide, in a random manner the sample thus selected into two portions of ten and fifty product units.
3The portion of ten units shall be used to test for the requirement of 5.3
The rest of the sample shall be used to test for the requirements of 4.3 and 5.2.
The toothpaste recovered from the extrusion test shall be well-mixed into a composite sample that will be used for all relevant tests on toothpaste.
A suitable number of empty tubes shall be taken from the sample to test for lead in paint.
Toothpaste shall be packaged in containers that shall neither show defects nor contaminate the toothpaste during the normal shelf life of the product.
The containers shall be further packed in individual carton boxes or other protective materials.
The immediate container (i.e. tube) and outer container shall be clearly marked with the following information:
(normative)
The material is brought into solution. The brown colour produced with aqueous hydrogen sulphide solution is matched with that produced with a standard lead solution.
Nessler tubes -100 ml capacity
Citric acid
Dissolve 0.1 g of bromophenol blue in 100 ml of rectified spirit.
Ammonium hydroxide
Dilute one volume of liquor ammonia (sp. gr. 0.92) with 10 volumes of water.
Thymol blue indicator
Dissolve 0.1 g of thymol blue in 100 ml of rectified spirit.
Potassium cyanide solution-10% (m / v).
Freshly prepared, saturated solution.
Dissolve 1.600 g of lead nitrate in water and make up the solution to exactly 1 000 ml. Transfer 10 ml of the solution by means of a pippete into a 1000 ml flask and dilute to the mark. One millitre of the final solution contains 0.01 mg of lead (Pb). The solution should be freshly prepared.
Accurately weigh 2.000 g of the toothpaste, in a platinum dish and incinerate for about 2 h at 525 °C to 550 °C. Cool, then add 10 ml of concentrated nitric acid and heat on a steam bath for 2 h. Evaporate off the nitric acid as far as possible, then add 5 ml of water and evaporate to dryness. Take
5the residue to fumes on a hot plate with three successive portions of hydrafluoric acid. Cool and dilute with water. Filter the solution, if necessary, with suction through a fine fritted glass filter and dilute the filtrate and washings to 200 ml in a graduated flask. This solution shall be used in the test in A.4.2 and B.B.2 (Arsenic).
Take in a beaker 100 ml of the solution prepared in A.4.1. Add 10 g of citric acid and adjust to pH 3.0 to 3.4 by adding ammonium hydroxide (to give a yellow purple colour with bromophonol blue indicator). Add about 10 mg of copper sulphate to act a co-precipitant. Precipitate sulphides by passing hydrogen sulphide until solution is saturated. Dissolve the sulphides, without previous washing, with 5 ml of hot dilute nitric acid, drawing solution through filter into the original flask; wash with hot water and collect the washing along with the solution in nitric acid. Boil to remove sulphureted hydrogen. Add 5 g of citric acid previously dissolved in water, make ammoniacal to pH between 8.5 and 10 (bluish) green to blue towards drop of thymol blue indicator) and add 5 ml of potassium cyanide solution. Transfer to a Nessler tube, add 10 ml of hydrogen sulphide solution, dilute to the mark and shake. Carry out a control test using 2 ml of standard lead solution and the same quantities of other reagents as used in test with the material.
The toothpaste shall be taken as not having exceeded the limit prescribed if the intensity of colour produced in the test with the material is not greater than that produced in the control test.
Lead in toothpaste is extracted with acid after low temperature ashing. The ammonium pyrrolidine dithiocarbamete (APDC) complex is formed, extracted into 4 – methylpentan-2-one and the lead is determined by atomic absorption spectroscopy.
All apparatus and reagents shall be lead free. Avoid contamination, particularly from atmospheric dust. To avoid volatilization of lead it is essential that ashing be carried out at a low temperature 450 °C is recommended, 500 °C maximum.
Apparatus
The following apparatus is required:
Beakers – 100 ml capacity, borosilicate glass or silica evaporating dishes.
Flasks – conical, I 00 ml capacity.
Funnels – separating 100 ml capacity.
Muffle furnace
Atomic absorption spectrophotometer and lead hollow cathode lamp
Pipette – Pasteur type
Pipette 10 mI
The following reagents shall be used and all shall be of recognized analytical reagent quality.
Acetone
Ethanol
64-methylpentan-2-one (isobutyl methyl ketone)
Nitric acid – concentrated
Nitric acid – 5 M
Ammonia solution – 5 M solution is recommended but a more dilute solution may be used if preferred
APDC one percent solution – Wash about 1. 5 g of ammonium pyrrolidine dithiocarbamate, (APDC) with 20 ml acetone in a P4 sintered glass crucible. Suck dry, weigh 1 g of the washed APDC and dilute to 100 ml with water.
Standard lead solution – (1 000 mg/kg lead)
Destruction of organic matter
Acid extraction of the ash
Determination of lead
NOTE The effect of the pH value on extraction of lead is indicated in table A.1
| pH | Peak height (mm) | |
|---|---|---|
| 0.5 μg/ml | 1.0 μg/ml | |
| 1 | 55 | 100 |
| 2 | 55 | 122 |
| 3 | 72 | 142 |
| 4 | 68 | 136 |
| 5 | 66 | 108 |
| 6 | 54 | 115 |
| 7 | 44 | 92 |
| 8 | 50 | 90 |
Submit aliquot portions of standard lead solution containing, 0 to 20 μg Pb to the procedure in A.4.4.5.3. Construct a new curve for each set of measurements.
Relate the test result to the calibration curve and calculate, μg Pb/9 (mg/kg).
8(normative)
Analytical balance
pH meter with appropriate glass/calomel electrode assembly
Stirrer
Weigh 5.0 g ± 0.5 g of toothpaste into a 100 ml beaker. Slurry with 20 ml ± 0.1 ml of distilled water. Determine the pH value of the slurry at a temperature of 23 °C ± 2 °C using the glass/calomel electrode assembly, about 3 min after the commencement of slurrying.
Report the pH value of the toothpaste
9(normative)
Oven
Transfer a suitable quantity of the composite sample of the tooth paste into each of two glass test tubes and stopper them. Heat the two tubes for 72 hours in an oven maintained at 45 °C ± 1 °C. Allow to cool and examine the contents visually for homogeneity and for signs of fermentation or other deterioration or both.
Report the result as pass or fail
10(normative)
Heat the tube of toothpaste at 45 °C ± 1 °C for ten days and then test as follows:Extrude a portion of the paste and examine it for visible contamination. Slit the tube, remove the paste and examine the surfaces of the tube for signs of corrosion, chemical attack or other damage. Disregard surface staining by colouring agents in the toothpaste.
Report the result as pass or fail.
11(normative)
Maintain the tube of toothpaste at 23 °C ± 2 °C for at least 2 hours and then test as follows: Remove the cap and invert the tube over a plastics beaker for which a tare has been recorded. Press-out the paste from the fold of the tube in the direction of the shoulder, holding the tube between thumb and index finger of the left and right hand.
When the tube is almost emptied, the mantle of the tube is to be pulled over an edge, removing the rest of the paste in the shoulder by nipping it off rectangular. The extrusion shall be carried out without the application of abnormal force.
Record the mass, m, of toothpaste extruded. Use a suitable method to determine the density of the toothpaste and hence calculate the volume of toothpaste extruded.
The extrusion, expressed as % (V/V) of the nominal volume, is given by the formula
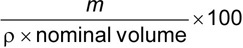
where
| m | is the mass of toothpaste extruded; |
| ρ | is the density of the toothpaste. |
(normative)
The fluorine derivative species in a sample of toothpaste (for total fluoride analysis) or in the aqueous extract of the toothpaste (for soluble fluoride analysis) is allowed to react with trimethylchorosilane under acidic conditions to produce trimethylfluorosilane. This derivative is extracted into toluene containing n-pentane as internal standard, and the organic phase is analysed by gas chromatography.
Use only reagents of analytical reagent grade and distilled or demineralised water.
Hydrochloric acid, concentrated.
n-Pentane solution in toluene (soln A).
0.6 g of n-pentane made up to 1 litre with toluene.
Standard fluoride solution (soln B).
An aqueous solution of sodium fluoride containing 1.0 mg F/mL.
Trimethylchlorosilane.
Gas chromatograph
A suitable gas chromatograph equipped with a flame ionization detector, and having a column of length about 1.5 m and nominal outside diameter 6 mm packed with 10%, methyl silicone (100%) on 80-100 mesh flux calcined diatomite, acid washed.
The recommended GC instrument conditions are as follows:
Temperatures – Column 50 °C
Injector 80 °C
Detector 100 °C
Carrier gas – Nitrogen, flow rate 20 ml/min
Centrifuge
Microsyringe, 1μl
Pipette 1 ml of the fluoride solution into a 50 ml plastics flask. Using a measuring cylinder, add 8 ml of concentrated hydrochloric acid, stopper the flask and mix the contents. Add from a burette2.0 ml of the trimethylchlorosilane.
Securely stopper the flask, mix well by shaking and allow to stand for 15 min to cool. Using a safety pipette, pipette 5 ml of the toluene solution of n-pentane into the flask and stopper. Mix thoroughly by shaking vigorously for 2 min. Transfer the contents of the flask to a centrifuge tube, stopper and centrifuge at 2500 r/min for 5 min. Inject 0.6 μl of the upper layer onto the column and allow the chromatogram to develop.
13Draw tangent base lines to the peaks of interest, and measure the heights of the trimethylfluorosilane and n-pentane peaks. ((Alternatively, determine the areas of the relevant peaks).
The response factor (k) is given by the formula
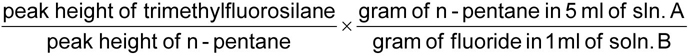
For soluble fluoride
Weigh accurately 5.0 ± 1.0 of toothpaste into a centrifuge tube. Add approximately 15 ml of water, slurry with a glass rod and centrifuge for 10 min. Decant the supernatant liquid into a 50 ml volumetric flask. Repeat this extraction process an additional two times and bring the volume of the supernatant liquid up to 50 ml with water.
Pipette 10 ml of the above solution into a plastics centrifuge tube. Add 8 ml of concentrated hydrochloric acid and immerse in a boiling water bath for I min and let stand for 10 min. Then coot under running water.
Add 1 ml of trimethylehlorosilane, cap, mix and let stand for 15 min. Pipette 5 ml of n-pentane solution into the tube, mix centrifuge and inject 0.6 μL of the upper layer.
The mass of fluoride, in grams, is given by the formula
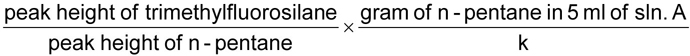
Weigh accurately 1.0 ± 0.1 g of toothpaste into a plastics centrifuge tube. Add several glass beads and 8 ml of concentrated hydrochloric acid in parts. Cap and shake, pausing several times to release pressure. Add 8 ml of water, cap and mix. Immerse in a boiling water bath for I min. Mix and let stand for 2 min, then cool under running water.
Add l ml of trimethylchlorosilane, mix and let stand for 15 min. Pipette 5 ml of n-pentane solution into the tube, mix, centrifuge and inject 0.6 gl of the upper layer.
The mass of fluoride, in grams, is given by the formula
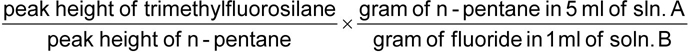
Expression of result
Report the results of total and soluble fluorine derivative of the sample tested as mg/kg of fluoride ion.
14(normative)
On 150-micron sieve
Place about 10 g of the toothpaste, accurately weighed, in a 100 ml beaker. Add 50 ml of water and allow to stand for 30 min, with occasional stirring until the tooth-paste is completely dispersed. Transfer to a 150-micron sieve and wash by means of a slow stream of running tap water and finally with a fine stream from a wash bottle until all the matter that can pass through the sieve has passed. Let the water drain from the sieve and then dry the sieve containing the residue in an oven. If there is any residue on the sieve, carefully transfer it to a tared watch glass and dry it to constant mass in an oven at 105 °C ± 2 °C.
Material retained on 150-micron
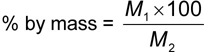
where
| M1 | is the mass in g of residue retained on the sieve; |
| M2 | is the mass in g of the material taken for the test. |
On 75-micron sieve
Weigh accurately about 10 g of the toothpaste and proceed as in G.1.2, using a 75-micron sieve. If there is any residue on the sieve carefully transfer it to a tared watch glass and dry it to constant mass in an oven at 105 °C ± 2 °C.
Calculate as in G.1.3
15(normative)
The modified Gutzeit method of test of Arsenic shall be employed in cases where arsenic content is not needed and only a knowledge of is desired. In cases where the actual arsenic content is to be determined, silver diethyldithiocarbamate method outlined in EAS 101 shall be followed.
Arsenic present in a solution of the material is reduced to arsine which is made to react with mercuric bromide paper. The stain produced is compared with a standard stain.
Dilute one volume of concentrated sulphuric acid with four volumes of water, and add to this 10 g of sodium chloride for each 100 ml of the solution.
Dissolve 84 g of ferric ammonium sulphate in water containing 10 ml of mixed acid and make up to one litre.
Dissolve 80 g of stannous chloride (SnCl2.2H2O) in 100 ml of water containing 5 ml of concentrated hydrochloric acid.
Carry out the test by adding into the Gutzeit bottle 2 ml of ferric ammonium sulphate solution, 0.5 ml of stannous chloride solution and 50 ml of the solution as prepared in EAS 100. For comparison, prepare a stain using 0.001 mg of arsenic trioxide.
16(informative)
MS 15, Specification for Toothpaste
BS 5136, Specification for Toothpastes
TZS 72, Toothpaste specification
17