
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.

EAS 14:2000
ICS 67.140
EAST AFRICAN COMMUNITY
© EAC 2000
First Edition 2000
vDevelopment of the East African Standards has been necessitated by the need for harmonizing requirements governing quality of products and services in the East African Community. It is envisaged that through harmonized standardization, trade barriers that are encountered when goods and services are exchanged within the Community will be removed.
In order to achieve this objective, the Community established an East African Standards Committee mandated to develop and issue East African Standards.
The Committee is composed of representatives of the National Standards Bodies in Partner States, together with the representatives from the private sectors and consumer organizations. Draft East African Standards are circulated to stakeholders through the National Standards Bodies in the Partner States. The comments received are discussed and incorporated before finalization of standards, in accordance with the procedures of the Community.
East African Standards are subject to review, to keep pace with technological advances. Users of the East African Standards are therefore expected to ensure that they always have the latest versions of the standards they are implementing.
© East African Community 2000 – All rights reserved*
East African Community
P.O. Box 1096
Arusha
Tanzania
Tel: 255 27 2504253/8
Fax: 255 27 2504255
E-mail: eac@eachq.org
Web: www.eachq.org
* © 2000 EAC — All rights of exploitation of any form and by any means reserved worldwide for EAC Partner States’ NSBs
viMargarine — Specification
This East African Standard specifies requirements, methods of sampling and test for margarine.
For the purpose of this East African Standard, the following definitions shall apply.
a food in the form of a plastic or fluid emulsion, which is mainly of the type water/oil, produced principally from edible fats and oils or mixtures thereof, which (may or may not have been subjected to a process of modification, and) are or are not mainly derived from milk
foodstuffs composed of glycerides of fatty acids of vegetable, animal or marine origin, hydrogenated or otherwise. They may contain small amounts of other lipids such as phosphatides, of unsaponifiable constituents and of free fatty acids naturally present in the fat or oil.
oils in which all or part of the following changes has been made, interesterification, hardening, fractional crystallization
any substance including a food additive, used in the manufacture or preparation of the food and present in the final product
any substance not normally consumed as a food by itself and not normally used as a typical ingredient of food, irrespective of its nutritive value, the intentional addition of which to food is for a technological (including organoleptic) purpose, in the manufacture, processing, preparation, treatment, packing, packaging, transportation or holding of such food results, or may be reasonably expected to result, directly or indirectly, in it or its by-products becoming a component of, or otherwise affecting the characteristics of such foods
packaged or made up in advance, ready for retail sale in a container or wrapper
1collection of packages of the same size, type and style, which have been manufactured and packaged under essentially the same conditions
any substance, not intentionally added to margarine, which is present in margarine as a result of production (including operations carried out in crop husbandry, animal husbandry and veterinary medicine), manufacture, processing, preparation, treatment, packing, packaging, transport or holding of margarine as a result of environmental contamination
Edible fats and/or oils, or mixtures of these (modified or not), vitamins, salt, sweetening matter proteins food additives, flavours, emulsifiers, water and/or milk and/or milk products, preservatives and anti oxidants.
Chemical characteristics of margarine shall conform to the requirements in Table 1.
| Characteristic | Requirement | Method of test |
|---|---|---|
| Fatty matter (m/m), minimum | 80 % | 9.1 |
| Water content (m/m), maximum | 16 % | 9.3 |
| Free fatty acid (as oleic acid) m/m, maximum | 0.3 % | 9.2 |
| Peroxide value, mill equivalents peroxide-oxygen/kg, maximum | (10) | ISO 3960:1977 |
Vitamins may be added to the margarine for the purpose improving nutritional value. When vitamins are added to margarine, the margarine shall comply with the limits set in 3.2.3.
Vitamins A shall be added to table margarine in such a way that complies with the requirements in table 2.
2| Nutrient | Requirements, RE1)/100g |
|---|---|
| Vitamin A, as retinol or as pro-vitamin A | 15-30 |
| 1)Conversion rates | |
Other vitamins may be added in such a way that the minimum levels of 15 % of the RDI per serving.
RDI given in US 500 shall be used.
NOTE 1 The determinations of vitamins A and D are considered as type tests and should be carried out for each formulation and when any change is made in the method of manufacture of margarine. Test methods described in the Official Methods of Test of the Association of Official Analytical Chemists (AOAC) should be used.
NOTE 2 A serving is the amount of food normally consumed in one helping. For margarine it should be 10 g.
Other ingredients include the following.
Sodium chloride, 0.1 % min, 5 % max.
Sugar, including any carbohydrate sweetening matter
Suitable edible proteins
Margarine shall be clean and free from rancidity, adulterants, pathogenic micro-organisms and mycotoxins especially aflatoxin, separated water and other foreign matter.
| Maximum level | |
|---|---|
| Beta-carotene | Limited by G.MP |
| Annatto | Limited by G.MP |
| Curcumin | Limited by G.MP |
| Canthaxanthine | Limited by G.MP |
| Beta-apo-8’-carotenal | Limited by G.MP |
| Methyl and ethyl ester of Beta-apo-8’-carotenoic acid | 25 mg/kg |
Natural flavours and their identical synthetic equivalents, except those which are known to represent a toxic hazard, and other synthetic flavours approved by the Codex Alimentarius Commission are permitted for the purpose of restoring natural flavours lost in processing or for the purpose of standardizing flavour, as long as the added flavour does not deceive or mislead the consumer by concealing damage or inferiority or by making the product appear to be of greater actual value.
| Maximum level | |
|---|---|
| Mono-and diglycerides of fatty acids | Limited by GMP |
| Mono-and diglycerides of fatty acids esterified with the following acids: – Acetic – Acetyltartaric – Citric – Lactic – Tartaric and their – sodium and calcium salts |
10 g/kg |
| Lecithins and components of commercial lecithin | Limited by GMP |
| Polyglycerol esters of fatty acids | 5 g/kg |
| 1,2-propylene glycol esters of fatty acids | 20 g/kg |
| Esters of fatty acids with poly-alcohols other than glycerol: – Sorbitan monopalmitate – Sorbitan monosterate – Sorbitan tristearate |
10 g/kg |
| Sucrose esters of fatty acids (including sucroglycerides) | 10 g/kg |
| Sorbic acid and its sodium, potassium and calcium salts | 1000 mg/kg individually or in combination |
| Benzoic acid and its sodium and potassium salts | expressed as the acid |
| 4.5.1 | Propyl, octly, and dodecyl gallates | 100 mg/kg individually |
| 4.5.2 | Butylated hydroxytoluene (BHT) | 75 mg/kg 4 |
| Butylated hydroxyanisole (BHA) | 175 mg/kg | |
| 4.5.3 | Natural and synthetic tocopherols | Not limited |
| 4.5.4 | Ascorbyl palmitate | 200 mg/kg individually or in combination |
| 4.5.5 | Ascorbyl stearate |
| Isopropyl citrate mixture | 100 mg/kg |
| 4.7.1 | Citric and lactic acids and their potassium and sodium salts | Limited by GMP |
| 4.7.2 | L-tartaric acid and its sodium and sodium potassium salts | Limited by GMP |
| 4.7.3 | Sodium hydrogen carbonate, sodium carbonate, sodium hydroxide | Limited by GMP |
Heavy metals
| Maximum Level | ||
|---|---|---|
| 5.1.1 | Iron (Fe) | 1.5 mg/kg |
| 5.1.2 | Copper (Cu) | 0.1 mg/kg |
| 5.1.3 | Lead (Pb) | 0.1 mg/kg |
| 5.1.4 | Arsenic (As) | 0.1 mg/kg |
Mycotoxins
Microorganisms, pathogenic microorganisms and mycotoxins especially aflatoxin
The product shall be prepared and handled under strict hygienic conditions by persons free from contagious and infectious diseases and only in premises maintained in thoroughly clean and hygienic conditions and having adequate and safe water supply and duly approved and licensed by the public health authorities concerned. All workers shall use clean, washable clothing, preferably white. Necessary precautions shall be taken to prevent incidental contamination of the product from soiled equipment or from personnel suffering from injury.
5To the extent possible in good manufacturing practice (GMP), the product shall be free from objectionable matter.
If margarine is manufactured, partly or wholly from edible fats or oils of animal or marine origin, the animals at the time of slaughter shall have been in good health and fit for human consumption as determined by a competent authority.
Organoleptic characteristics — The taste, odour and colour of the product shall be pleasant and typical. The product shall have no bitter taste or rancid odour that indicates spoilage. No organism such as mould and other deterioration organisms shall be visible in the product. The appearance of a cut surface shall be uniform and typical.
Margarine when sold by retail shall be pre-packed (in clean, dry, new and non-absorbent container or wrapper) and may be sold in pack of any shape, which has no adverse influence upon the composition of the product, its properties or appearance. Containers shall be free from other products that may lead to contamination and alter the quality, composition, odour and taste of margarine. Containers shall be airtight and shall be provided with tamper-proof seals and closures. Containers shall protect margarine from light, prevent loss of moisture and shall preclude contamination with proliferation of micro-organisms in margarine during storage and transport. Containers shall be free of pinholes in order to avoid corrosion and rust.
Margarine shall be packaged in formed aluminium or plastic tubs and trays or in enamelled
In addition to the relevant requirements of EAS 38, the following specific provisions apply:
The name of the food — The product shall be designated “margarine” and all products designated, as “margarine” shall conform to this standard.
List of ingredients — Made from edible vegetable oils. A complete list of ingredients shall be declared on the label in descending order of proportion in accordance with EAS 38.
Net contents — The net contents shall be declared by weight in metric units ("System International” units).
Name and Address — The name and address of the manufacturer, packer, distributor, importer, exporter or vendor of the product shall be declared.
Country of origin — The country of origin of the product shall be declared. When the product undergoes processing in a second country, which changes its nature, the country in which the processing is performed shall be considered to be the country of origin for the purposes of labelling.
Labelling prohibitions — No reference shall be made to the presence of milk fat or butter in margarine except in a complete list of ingredients.
No reference shall be made, other than in a complete list of ingredients, to the presence of any vitamin in margarine unless the name and quantity of the vitamin is stated on the label.
6Lot identification — Each container shall be embossed or otherwise permanently marked in code or in clear to identify the producing factory and the lot.
Date marking — Month and year of manufacture. The date of minimum durability of the food shall be declared in clear.
In addition to the date, any special conditions for the storage of the food should be indicated if the validity of the date depends thereon.
Added nutrients shall be declared on the label in accordance with US 500. Claims may be made in accordance with US 508 and DUS 566.
Heat the specimen (the entire package or part of it) in a container immersed in a water bath in order to soften it. The temperature of the bath shall be as low as possible and shall not exceed 39 °C. Excess heating shall be avoided, because it may cause visible separation of the emulsion. Mix while heating in order to undo any separation that has started and to obtain uniformity and fluidity. The texture is optional when an emulsion is present and the specimen is fluid to a satisfactory degree.
Remove the container from the water bath, mix well (this can also be done by a mechanical mixer) until the specimen cools again and a creamy consistency is obtained. Weigh a required test quantity immediately.
Determine the fatty matter content in the test quantity used previously for determination of the water content. Extract the test quantity in anhydrous alcohol-free either or in petrol, of maximum 65 °C boiling temperature. Filter the ethenic extraction liquid and transfer it to a weighed vessel. Evaporate and dry in an oven at a temperature of 100 °C until constant weight is obtained (M g). Calculate the fatty content (F percent by weight) from the formula:
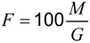
where:
F is the percent by weight of fatty matter; G is the weight of the test quantity taken for the determination of water content (see 9.3); and M is the constant weight obtained after drying.
From the prepared material (see 9.1), weigh, to an accuracy of 0.01 g, a test quantity weighing G g; (15 g - 2.5 g for salted margarine or 2 g - 6 g for unsalted margarine). Weigh in a shallow vessel, of more
7than 5 cm in diameter. Dry in an oven, of 100 °C temperature, until constant weight is obtained (P g). Cool in a desiccator and weigh. Calculate the water content (H percent by weight) from the formula:
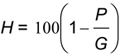
where
H is the water content percent; P is the constant weight; and G is the weight of sample.
Weigh to an accuracy of 0.01 g a test quantity of approximately 50 g in weight (W g). Dissolve the test quantity in hot petroleum ether in a beaker of 250 ml capacity.
Filter though ordinary paper, then rinse the sediment several times with petroleum ether until all signs of fat disappear from the filter paper. Transfer the paper with the sediment to a Kjeldahl bottle and determine the nitrogen content (N) by means of the Kjeldahl method.
Calculate the content of fat-free milk solids (N) in percent of weight from the formula:
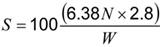
where:
S is the fat-free solids; W is the weight of the test sample; and N is the nitrogen content of the margarine expressed as percent by weight
The assay sample is saponified with ethanolic potassium hydroxide and Vitamin A is extracted with petroleum ether. After reaction with antimony trichloride, the blue colour formed is measured in a colorimeter at 610 nm to 620 nm. Calculation of Vitamin A content is carried out with reference to a calibration curve.
Photoelectric colorimeter with a direct reading deflection type galvanometer, suitable for measuring transmittance or absorbance at 610 nm to 620 nm
8Vitamin A reference standard — solution of crystalline Vitamin A of accurately known strength
Absolute ethanol — Purified as follows:
Reflux absolute ethanol for 30 min in the presence of 10 g caustic potash and 10 g aluminium powder per litre of ethanol. The mixture is then distilled in an all-glass apparatus with a fractionating column of 50 cm to 60 cm length. Collecting middle fraction of the distillate, discard the first and the last fraction, each amount to 5 % of the total volume. The ethanol thus obtained should have the same optical absorption as glass distilled water in ultraviolet region.
Potassium hydroxide solution, 50 % (v/v)
Ether — peroxide-free, redistilled ether may be maintained from peroxide by adding washed zinc foil approximately 80 cm2 per litre, cut in strips long enough to reach at least half way up the container. The zinc strips previously shall have been completely immersed in dilute acidified copper sulphate solution for one minute and subsequently washed with water.
Sodium sulphate, anhydrous, granular — It shall not absorb Vitamin A under conditions of use, and 10 % solution shall not be acid to methyl red indicator solution.
Chloroform — This may be purified as follows:
Wash the chloroform trice with fresh 10 % aqueous solution of sodium thiosulphate in a separate funnel. Dry the chloroform with anhydrous calcium chloride and filter. Distil the chloroform over anhydrous sodium thiosulphate in an all-glass apparatus with a fraction of the distillate, discard the first 10 % and last 10 % of the distillate.
Anhydrous sodium thiosulphate may be prepared from crystalline thiosulphate by careful heating between 105 °C and 110 °C, and storing in a desiccator.
Antimony trichloride in solution — Prepare by dissolving 113.4 g antimony trichloride in 300 ml to 400 ml of chloroform. Add 5 g of anhydrous calcium chloride and filter while hot. Dilute the filtrate to 500 ml with chloroform.
Adequate precautions shall be taken to protect the Vitamin A content in the flask from direct actinic light.
Evaporate a suitable aliquot of the ether solution of the unsaponifiable extract to about 5 ml. Evaporate off the remaining ether at low heat under reduced pressure. Take up the residue in sufficient chloroform so that after addition of antimony trichloride solution an absorbance of about 0.8 in the photoelectric colorimeter is obtained.
From this stock solution of the standard, make a series of dilutions in chloroform to give absorbance values of 80 %, 60 %, 40 % and 20 % of the original absorbance. Determine absorbance of the blue colour formed when one millilitre aliquot of each of these five solutions plus one millilitre of chloroform with a drop of acetic anhydride is treated with the volume of the antimony trichloride solution, that is, suitable for the operation and hereinafter, referred to as the `fixed volume’. The blank is adjusted to 100 % transmittance using a tube containing 2 ml of chloroform and the fixed volume of the antimony trichloride solution.
Using a rectangular co-ordinate paper, plot the five absorbances obtained against known quantities of Vitamin A and draw up the best smooth curve from the origin through these points. Do not attempt to draw straight line unless the curve is in fact a straight line with the origin at zero. For those instruments that provide other than straight-line curve, check this curve at frequent intervals. For these instruments that do provide straight lien calibration curve, make reading of the reference solution with each set of sample readings to establish the curve. In the latter case reestablish the calibration curve whenever variation in the reagent or other variable in procedure occurs.
Weigh accurately a quantity of the material (not more than 5 g) containing 20 to 45 i.u of Vitamin A, then proceed as (b) and (c) and obtain the residue in a definite volume of chloroform so that 2 ml of the chloroform solution with the fixed volume of the antimony trichloride solution would give absorbance of about 0.5 to 0.2. Set the instrument at 100 % transmittance with 2 ml of chloroform and flexed volume of the antimony trichloride as blank. Place the tube containing 2 ml of the chloroform solution of the residue, add a drop of acetic anhydride and then add rapidly the `fixed volume’ of antimony trichloride solution with the help of vacqupet or a similar device.
Record the maximum calorimetric reading. Determine Vitamin A from the standard curve and calculate units of Vitamin A per 100 g of the sample.
NOTE Care should be taken that the readings are noted within 15 s after the addition of antimony trichloride solution.
From the material prepared (see 9.1) weigh, to an accuracy of 0.01 g, a test quantity of weight G g (approximately 5 g) to conical bottle of 250 ml capacity and add 100 ml of boiling water. Hold for approximately 10 min agitating from time to time until the temperature is lowered to 50 °C - 55 °C.
10Add 2 ml of potassium chromate (K2CrO4) solution (as indicator) and titrate with silver nitrate (AgNO3), 0.1 M until an orange-brown colour appears lasting for approximately 30 s.
Calculate the salt content A (expressed in percent of sodium chloride) from the formula:
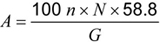
where:
A is the percentage of sodium chloride N is the normality of the silver nitrate solution n is the quantity of silver nitrate solution used for the titration (cc) G is the quantity of the fat taken.
Spectrophotometer, TLC plates, chromatographic tank, steam distillation flasks.
MgSO4. 7H2O, H3PO4, CHCl3 ether, petroleum ether (b.p 60 °C – 80 °C), CaCl2, FeCl3 of ferric alum, thiobarbituric acid, potassium dichromate-sulphuric acid, potassium sorbate.
TLC plates — 10 g Kieselguhr G and 10 g Kieselgel GF 254 + 45 ml H2O (five plates of 0.25 mm thickness). Air-dry for 10 min then in oven at 100 °C for 1 h.
Thiobarbituric acid — Dissolve 0.5 g of 2-thiobarbituric acid (TBA) in 20 ml of water and 10 ml of 1 N NaOH in a 100 ml volumetric flask. After dissolving, add 11 ml of 1 N HCl to make to the volume. The 2-thiobarbituric acid shall be made fresh daily. The reagent remains relatively unchanged for a 6-h period, but after one-day absorbance values are lower, resulting in erroneous analytical results. The amount of loss is about 40 % after 14 days and 100 % after 30 days at ambient temperature.
Potassium dichromate-sulphuric acid — Mix M/600 K2Cr2O7 and 0.15 M H2SO4 in 1:1 proportions.
Potassium sorbate — Dissolve 250 mg of potassium sorbate in water in a 250 ml volumetric flask and dilute to the mark.
Benzoic acid and benzoate
11Blend entire unit sample in high-speed blender to homogeneous mixture. Quantitatively transfer about 50 g - 60 g blended sample (accurately weighed) to 800 ml Kjeldahl flask with a small portion of water if necessary. Add 200 g MgSO4 and 25 ml 6H2PO4. Wash down the neck of the flask with H2O until the total volume is 350 ml - 375 ml.
Steam-distil the sample directly into 1 litre separator containing 50 ml NaOH solution (4 g/100 ml); collect 725 ml - 750 ml distillate in 90 min or longer by adjusting the power stats. (Distillation rate is very critical; typical drop time is 50 drops/20 s). Rinse the condenser with about 20 ml of water. Acidify distillate to litmus with about 20 ml HCl. Extract with 1 ml × 100 ml and 4 ml × 50 ml portion of CHCl3-ether (2+1). Shake each extraction vigorously for one or more minutes and collect extracts in a 600 ml beaker. Evaporate the combined CHCl3-ether extracts carefully on a steam bath, using gentle air currents to about 25 ml, washing down sides of the beaker occasionally with CHCl3-ether. (Do not let extracts go to dryness). Transfer to a 50 ml volumetric flask, wash the beaker with small portion of CHCl3-ether and transfer the washings to the volumetric flask. Dilute the volume with CHCl3-ether for TLC analysis.
Spot 100-microlitre-sample solution, using microlitre Hamilton Syringe twice under gentle air draught on prepared plate. Also, spot 100-microlitre standard benzoic solution (50 mg/50 ml alcohol). Spot sample(s) and standard about 25 mm from bottom edge of plate and 40 mm apart, beginning 25 mm from side edge. Place the plate in chromatographic chamber containing 250 ml - 300 ml mobile solvent; n-hexane-HOAc (96 + 4), and develop chromatogram for 10 cm. Remove and air-dry for 5 min. Observe under UV short wave radiation (254 nm). The sample and benzoic acid appear as dark blue-purple spots on light fluorescent background.
With pencil, circle spots 12.5 mm from outer edge of each spot. (This gives exact location of each acid when plate is removed from radiation). Scrape off encircled areas with steel spatula onto a piece of Glassine paper and carefully transfer to 10 ml volumetric flask. From unused portion of plate, scrape off area about equal to that used for sample into 10 ml volumetric flask to serve as blank to each, add 7 ml alcohol, stopper and shake for 30 s. Dilute to volume with alcohol, transfer to a centrifuge tube, and centrifuge until clear (ca 5 min) at high speed. Decant the clear supernatant solution into a 5 cm micro cell and scan on a recording spectrophotometer from 310 nm to 250 nm against blank. Compare sample with standard.
Percent benzoic acid = [(A sample/A std.) × (g std./50 ml) / (g sample/50 ml)] × 100 % sodium benzoate = Percent benzoic acid × 1.180
where
12
A sample is the absorbance of sample A std. is the absorbance of standard g sample is the weight of sample g std. is the weight of standard.
For determining the standard curve, use margarine that has not been heated with potassium sorbate. Blend 10 g of fat for 2 min in 90 ml of water in a blender. Add 10 g of slurry to each of five 250 ml volumetric flasks. Add 0.25 ml, 0.5 ml, 0.75 ml and 1.0 ml of the standard sorbate solution and make up to volume. In each case, filter and add 2 ml of the filtrate to a test tube containing 2 ml of potassium dichromate-sulphuric acid solution. Heat for 5 min at 100 °C in an oil bath.
Add 2 ml of thiobarbituric acid to the tube and allow it to remain in the bath for an additional 10 min. Remove and cool quickly in running tap water. Measure the absorbance at 530 nm in a spectrophotometer; use water as a blank (for 100 % transmission). Plot absorbance versus µg potassium sorbate × 125 = ppm potassium sorbate in the fat.
Determine the counts of bacteria yeast and mould in accordance with ISO 4832:1991, General Guidance for Microbiological Examinations and ISO 7218: 1985, General Guidance for the Enumeration of Micro-organisms — Colony Count Technique at 30 °C
Determine lead, copper and arsenic according to the methods described in CAC/RM 54-1974 of Codex Alimentarius Commission.
Determine iron by employing the method described in CAC/RM 14-1969 of Codex Alimentarius Commission
According to the AOAC (1965) method (Official Methods of Analysis of the AOAC, 1965, 39.116 — 39.129 Vitamin D) results are expressed as IU Vitamin D per kg.
According to the FAO/WHO Codex Alimentarius Method CAC/RM 18-1969, Determination of Vitamin E (Tocopherols) Content. Results are expressed as milligrammes of each tocopherol per kilogramme of the product.
13