
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.

EAS 127:1999
ICS 97.040
EAST AFRICAN COMMUNITY
© EAC 1999
First Edition 1999
i| Page | ||||
| Foreword | iv | |||
| 1 | Scope | 1 | ||
| 2 | Normative references | 1 | ||
| 3 | Requirements | 1 | ||
| 4 | Packing and marking | 2 | ||
| 5 | Sampling | 2 | ||
| 6 | Tests | 2 | ||
| Annex A (informative) List of suggested builders and additives | 3 | |||
| Annex B (normative) Methods of test for synthetic detergents | 4 | |||
| B.1 | Quality of reagents | 4 | ||
| B.2 | Qualitative identification of non-soapy detergents | 4 | ||
| B.2.1 | General | 4 | ||
| B.2.2 | Apparatus | 4 | ||
| B.2.3 | Reagents | 4 | ||
| B.2.4 | Procedure for identification of soapy and non-soapy detergents | 4 | ||
| B.2.5 | Procedure for identification of the type of non-soapy detergents | 5 | ||
| B.3 | Qualitative identification of alkyl aryl sulfonates | 5 | ||
| B.3.1 | General | 5 | ||
| B.3.2 | Apparatus | 5 | ||
| B.3.3 | Reagents | 6 | ||
| B.3.4 | Procedure | 6 | ||
| B.4 | Determination of moisture and volatile matter content | 6 | ||
| B.4.1 | General | 6 | ||
| B.4.2 | Apparatus | 6 | ||
| B.4.3 | Procedure | 6 | ||
| B.4.4 | Calculation | 6 | ||
| B.5 | Determination of active ingredient | 7 | ||
| B.5.1 | General | 7 | ||
| B.5.2 | Apparatus | 7 | ||
| B.5.3 | Reagents | 7 | ||
| B.5.4 | Procedure | 8 | ||
| B.6 | Determination of matter insoluble in alcohol | 10 | ||
| B.6.1 | General | 10 | ||
| B.6.2 | Apparatus | 10 | ||
| B.6.3 | Reagents | 10 | ||
| B.6.4 | Procedure | 10 | ||
| B.6.5 | Calculation | 11 | ||
| B.7 | Determination of phosphates in matter insoluble in alcohol | 11 | ||
| B.7.1 | General | 11 | ||
| B.7.2 | Apparatus | 11 | ||
| B.7.3 | Reagents | 11 | ||
| B.7.4 | Procedure | 12 | ||
| B.7.5 | Calculation | 12 | ||
| B.8 | Determination of pH | 13 ii | ||
| B.8.1 | General | 13 | ||
| B.8.2 | Apparatus | 13 | ||
| B.8.3 | Reagents | 13 | ||
| B.8.4 | Procedure | 13 | ||
| B.9 | Determination of non-detergent organic matter | 13 | ||
| B.9.1 | General | 13 | ||
| B.9.2 | Apparatus | 14 | ||
| B.9.3 | Reagents | 14 | ||
| B.9.4 | Procedure | 14 | ||
| B.9.5 | Calculation | 14 | ||
| B.10 | Determination of matter insoluble in water | 15 | ||
| B.10.1 | Procedure | 15 | ||
| B.10.2 | Calculation | 15 | ||
| B.11 | Determination of carboxymethylcellulose | 15 | ||
| B.11.1 | Principle | 15 | ||
| B.11.2 | Reagents | 15 | ||
| B.11.3 | Apparatus | 16 | ||
| B.11.4 | Procedure | 16 | ||
| B.11.5 | Calculation | 16 | ||
| Annex C (normative) Sampling procedure for powder detergents | 17 | |||
| C.1 | General requirements | 17 | ||
| C.2 | Scale of sampling | 17 | ||
| C.3 | Preparation of gross samples, test sample and reference sample | 18 | ||
| C.3.1 | Gross sample | 18 | ||
| C.3.2 | Test sample | 18 | ||
| C.3.3 | Referee samples | 19 | ||
| C.4 | Number of tests | 19 | ||
| C.5 | Criteria for conformity | 19 | ||
| C.5.1 | For individual samples | 19 | ||
| C.5.2 | For composite sample | 20 | ||
| Bibliography | 21 | |||
Development of the East African Standards has been necessitated by the need for harmonizing requirements governing quality of products and services in the East African Community. It is envisaged that through harmonized standardization, trade barriers that are encountered when goods and services are exchanged within the Community will be removed.
In order to achieve this objective, the Community established an East African Standards Committee mandated to develop and issue East African Standards.
The Committee is composed of representatives of the National Standards Bodies in Partner States, together with the representatives from the private sectors and consumer organizations. Draft East African Standards are circulated to stakeholders through the National Standards Bodies in the Partner States. The comments received are discussed and incorporated before finalization of standards, in accordance with the procedures of the Community.
East African Standards are subject to review, to keep pace with technological advances. Users of the East African Standards are therefore expected to ensure that they always have the latest versions of the standards they are implementing.
© East African Community 1999 – All rights reserved*
East African Community
P.O. Box 1096
Arusha
Tanzania
Tel: 255 27 2504253/8
Fax: 255 27 2504255
E-mail: eac@eachq.org
Web: www.eachq.org
* © 1999 EAC — All rights of exploitation of any form and by any means reserved worldwide for EAC Partner States’ NSBs
ivThere are two main groups of detergents, namely, the soaps and non-soapy detergents. In order to guide the production of non-soapy detergents of well-defined quality and also to safeguard consumer interests, it has been felt desirable to formulate this standard.
Non-soapy detergents are of three types: anionic, cationic and non-ionic. Anionic non-soapy detergents of the alkyl aryl type, such as dodecylbenzene sulfuric acid, are now produced in fairly large quantities and hence priority has been given for the standardization of this material.
v viPowdered laundry detergents for household use — Specification
This East African Standard prescribes the requirements and methods of sampling and test for synthetic anionic detergents for household use based predominantly on the use of alkyl aryl sulfonates.
The following referenced documents are indispensable for the application of this document. For dated references, only the edition cited applies. For undated references, the latest edition of the referenced document (including any amendments) applies.
EAS 123:1999, Distilled Water – Specification
Description — The active ingredient shall be the sodium salt of alkyl aryl sulfonic acid. The formulation may contain one or more of the builders or additives given in Annex A. The active ingredient used shall be biodegradable.
Form — The detergent shall be homogenous powder, free flowing, free from visible impurities, abrasives and organic solvents and readily soluble in water.
Effect on health — The detergent shall not be irritating to the normal skin and shall not contain any ingredients in a quantity that is toxic to human beings.
The material shall be a free flowing powder, and shall not give an unpleasant odour and shall give good lather.
The material shall also comply with the requirements given in Table 1, when tested by the appropriate method prescribed in Annex B, as indicated in column 4 of the table.
Odour — Neither the detergent nor a solution of the detergent in hot water (at 60 °C ± 2 °C) shall have objectionable odour. Perfume may be added, in which case, the perfume shall not change its fragrance nor develop an objectionable odour during storage at ambient temperature for six months.
Colour — The detergent may be coloured provided that the colouring is uniform and does not change appreciably during storage.
1Storage properties — When stored or transported under normal conditions in the original container, the powder shall not cake into lumps.
| S/No | Characteristics | Requirements | Method of test (Ref. To Col. No. in Annex B) |
|---|---|---|---|
| 1 | Moisture and volatile matter content at 150 °C, % by mass, max | 13 | B.4 |
| 2 | Active ingredient, % by mass, min | 18 | B.5 |
| 3 | Matter insoluble in alcohol, % by mass, max | 70 | B.6 |
| 4 | Phosphate (expressed as sodium tripolyphosphate), % by mass of matter insoluble in alcohol, min | 20 | B.7 |
| 5 | pH of 1 % solution (m/v), at 30 °C | 9 to 11 | B.8 |
| 6 | Non-detergent organic matter, % by mass, max | 1.0 | B.9 |
| 7 | Matter insoluble in water, % by mass, max | 0.5 | B.10 |
| 8 | Sodium carboxymethyl cellulose, % by mass, min | 2.0 | B.11 |
The material shall be supplied in suitable well-closed containers, as agreed to between the purchaser and the supplier.
The containers shall be securely closed and legibly marked with the following information:
Representative samples of the material shall be drawn as prescribed in Annex C.
Tests shall be carried out as prescribed in Annex B. Reference to the relevant clauses are given in column 4 of Table 1.
2(informative)
(normative)
Unless otherwise specified, chemicals of analytical grade and water, distilled quality, in accordance with EAS 123:1999, Distilled Water – Specification, shall be employed in all the tests.
It is recommended that the quantitative examination of a sample is preceded by a qualitative identification of the type of non-soapy detergent present. The procedure given in B.2.4 permits ascertaining whether the materials is based on a soap or a non-soapy detergent The method described in B.2.5 enables the identification of the type of non-soapy detergent, that is, whether it is cationic anionic or non-ionic.
Measuring cylinder, 100-ml capacity; glass-stoppered
Test Tubes
Hydrochloric acid, 50% (m/v) solution
Methyl orange indicator solution, 0.1 % (m/v) solution.
Methylene blue reagent — dissolve 0.5 g of methylene blue in distilled water and make up the volume to 100 mL. To 6 ml of this solution, add 120 mL of 1 M mol sulfuric acid and 50 g of anhydrous sodium sulfate, and make up the volume to 1 L with distilled water.
Cetyl dimethyl benzyl ammonium chloride solution, 0.2 % (m/v) solution
Sodium lauryl sulfate solution, 0.2 % (m/v) solution.
Take about 0.1 g of the sample in a test-tube, add about 20 ml of water and shake well until dissolution is complete. Add a drop of methyl orange indicator solution, and make it just acidic by
4adding a few drops of hydrochloric acid solution. If the lather is destroyed and fatty acids separate out, then the material is based on soap. If the lather persists, then the active matter is non-soapy detergent.
Dissolve about 0.1 g of the sample in about 20 ml of water, and take 10 ml of this solution in a measuring cylinder. Add 10 ml of methylene blue reagent and 15 ml of chloroform, shake well and allow to stand. Observe whether the colour is in the chloroform layer or the aqueous layer.
If the colour is initially in the chloroform layer (see B.2.5), add 0.1 ml of cetyl dimethyl benzyl ammonium chloride solution, and shake well and allow to stand. If the colour is regained in the chloroform layer, the active matter is anionic. If the colour is transferred to the aqueous layer, the active matter is non-ionic.
If the colour is initially in the aqueous layer (see B.2.5), add 0.1 ml of sodium lauryl sulfate solution, and shake well and allow to stand. If the colour is retained in the aqueous layer, the active matter is cationic. If the colour is transferred to the chloroform layer, the active matter is nonionic.
The detection depends on:
The active matter is fused with potassium hydroxide to convert it to the corresponding phenol. The phenol is then coupled with diazotized sulfanilic acid and the formation of a red dye confirms its presence.
Wide-mouthed flat bottomed flask, 150-ml capacity
Hydrochloric acid solution, approximately 3 M
Sodium hydroxide solution, approximately 2 M
Gently fuse a little of the material with solid potassium hydroxide in a nickel crucible, exercising great care to avoid charring. When the melt is quite clear, cool it, dissolve in distilled water, and transfer the solution to a separating funnel. Acidify with hydrochloric acid, extract with diethyl ether and run off the aqueous phase. Wash the other phase with water until free from mineral acid, and then transfer it to a flask. Remove the ether on a steam bath and dissolve the residue in sodium hydroxide solution.
Diazotize 0.05 g of sulfanilic acid by adding 0.1 g of sodium nitrite, and a few drops of hydrochloric acid to a solution in water, and pour it in the alkaline solution of the phenol (see B.3.4 1). The formation of a red dye indicates the presence of an aromatic ring and shows that the sulfonic acid was attached to the nucleus.
Moisture and volatile matter is determined by the oven method.
Porcelain or silica dish, 6 cm to 8 cm in diameter and 2 cm to 4 cm in depth
Desiccator, containing an efficient desiccant, such as phosphorus pentoxide
Air-oven, preferably electrically heated, with temperature control device
Weight accurately about 5 g of the material into a dry tared dish, and dry to constant mass in an air-oven at a temperature of 105 °C ± 1 °C. Cool in a desiccator and weigh. Constant mass shall be considered to have been attained when successive heating for one-hour periods shows a difference of not more than 5 mg in the net loss in mass.

where
m is the mass in g of the material taken for the test; and m0 is the mass in g of the material after drying
Active matter, namely, the sodium salt of sulfonated alkyl benzene is first separated from inorganic salts, non-detergent organic matter alkylolamide. It is then neutralized to phenolphthalein, evaporated to dryness, extracted with ethyl alcohol, dried and weighed. Finally, the weighed extract is corrected for the presence of sodium choride and alkali carbonates.
Beaker, 150-ml and 1 000-ml capacity
Buchner flask, 500-ml capacity, fitted with sintered glass filter funnel (porosity 4)
Separating funnel, 1 000-ml capacity
Wide mouthed flat-bottomed flask, 200-ml capacity
Air-oven, preferably electrically heated, with temperature control device
Ethyl alcohol, 30 %, 90 % and absolute (by volume)
Standard sulfuric acid, approximately 0.1 M
Standard silver nitrate solution, approximately 0.1 M
Phenolpthalein indicator, 1 % solution in 95 % (by volume) ethyl alcohol
Methyl orange indicator, 0.1 % (m/v)
7Nitric acid, concentrated, specific gravity, 1.42g/l
Standard ammonium thiocyanate solution, approximately 0.1 M
Ferric ammonium sulfate indicator, saturated solution
Caustic soda solution, 10 % (m/v)
Weigh accurately about 5 g of the material for products containing about 20 % active matter and correspondingly less for products of higher active matter content. Proceed as described in the determination of matter insoluble in alcohol (see B.6.4). After filtering and washing the residue with hot ethyl alcohol, evaporate the combined filtrate to a small bulk in an evaporating basin.
Dilute the evaporated filtrate (see B.5.4.1) with 50 ml of 90 % ethyl alcohol, and transfer to a separating funnel. Rinse the evaporating basin once with 50 ml of 90 % ethyl alcohol and then four times with 50-ml portions of distilled water. Add each wash in turn to the separating funnel. Add 150 ml of diethyl ether, swirl gently to ensure adequate mixing and allow the two phases to separate. Run off the aqueous alcoholic layer into a second separating funnel, and extract twice with 75-ml portions of diethyl ether. Transfer the aqueous alcoholic phase into a beaker, and combine the three ether extracts.
Take the combined ether extracts in a clean separating funnel. Wash three times with successive 50-ml portions of distilled water until the phase is free from alcohol; usually, 7 to 10 water washes are necessary.
Combine the washings and rinsings from (B.5.4.2.2) with the aqueous alcoholic phase obtained in (B.5.4.2.1).
Transfer the combined aqueous alcoholic phase and washings from B.5.4.2.3 to a porcelain basin. Neutralize to phenolphthalein and evaporate on a steam bath until the volume is reduced to about 25 ml. Add an equal volume of absolute alcohol and evaporate to dryness. The solution shall remain just pink to phenolphthalein throughout evaporation.
To ensure that the residue is completely anhydrous, add 30 ml of absolute alcohol and again evaporate to dryness. Extract the residue with 30 ml of hot 96 % ethyl alcohol, stirring and breaking up the solid matter in the dish with a glass rod. Allow the solid matter to settle and decant the hot alcoholic solution through a sintered glass filter fitted to a Buchner flask to which suction is applied. Extract the residue in the dish with six further consecutive 30-ml portions of hot 96 % ethyl alcohol. Pass each extract in turn through the sintered glass filter. Finally, wash the residue in the sintered glass filter three times with about 20 ml of hot 96% ethyl alcohol from the jet of a wash bottle.
Transfer the filtrate and washings in the Buchner flask to a tared wide-mouthed flat-bottomed flask, evaporate nearly to dryness on a water-bath, and drive off the remaining solvent by
8directing a gentle stream of dry air into the flask whilst continuously rotating the latter on the water-bath. A thin film of active matter, easy to dry is thereby obtained. Add 10 ml of acetone, evaporate and remove the last trace of solvent as described above, cool in a desiccator and weigh.
Heat the flask for not more than 5 min in air-oven at a temperature of 100 °C ± 1 °C, gently blow out with a current of air, cool and re-weigh. Repeat this drying process until the difference between two successive weighings does not exceed 3 mg. Record this mass as m.
The extract obtained in B.5.4.3.4 contains the active matter, some sodium chloride and possibly a trace of alkali carbonates, which may have passed through the filter In the presence of the detergent.
Dissolve the extract (see B.5.4.3.4) in cold distilled water, add a few drops of methyl orange indicator and titrate with standard sulfuric acid to the methyl orange end point.
Calculation
Mass in g of sodium carbonate, m2 = 0.106 VM
where
V is the volume in ml of standard sulfuric acid solution used; and M is the molarity of the standard sulfuric acid solution.
Reserve the solution for the estimation of chlorides.
To the solution remaining after the estimation of alkali carbonates (see B.5.4.4.3), add 2 ml of concentrated nitric acid and 20 ml of standard silver nitrate solution. Add 3 ml of nitro-benzene and shake vigorously. Titrate with standard ammonium thiocyanate solution using ferric ammonium sulfate as indicator.
Calculation — mass in g of sodium chloride,
m3 = 0.0585(20M − VM1)
where
9
M is the molarity of the standard silver nitrate solution; M is the volume in ml of standard ammonium thiocyanate solution used; and M1 is the molarity of the standard ammonium thiocyanate solution.
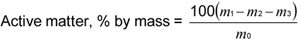
where
m1 is the mass in g of the alcoholic extract (see B.5.4.3.4); m2 is the mass in g of sodium carbonate (see B.5.4.4.2); m3 is the mass in g of sodium chloride (see B.5.4.5.2); and m0 is the mass in g of the material taken for the test.
By matter insoluble in alcohol is generally meant the inorganic salts, such as phosphates, sulfates, silicates and carbonates, which are usually present in non-soapy detergent preparations. These can be separated from active matter, non-detergent organic matter, etc, by extracting the material with 96 % ethanol.
Beaker, 150-ml capacity
Buchner flask, 500-ml capacity, fitted with sintered glass filter funnel (porosity 4)
Air-oven, preferably electrically heated, with temperature control device
Ethyl alcohol, freshly boiled, 96% or higher (by volume)
Weigh accurately about 5 g of the material into a beaker, and digest with 50 ml of ethyl alcohol by heating on a steam bath for about 2 min. Stir and break up any hard lump with a glass rod flattened at one end. Allow the solid matter to settle and decant the hot alcoholic solution through a sintered glass filter funnel fitted to a Buchner flask, to which suction is applied. Repeat the alcoholic digestion in a similar manner with five further consecutive 30-ml portions of boiling ethyl alcohol. Filter each extract in turn through the same sintered glass funnel and, finally, wash the residue several times with hot ethyl alcohol to remove all the alcohol solubles. Dry the sintered glass funnel with the residue in an air-oven at a temperature of 105 °C ± 2 °C until a constant mass is obtained.
10Even after digestion with five 30-ml portions of boiling ethyl alcohol, the alcohol insoluble portion may sometimes be found to be sticky. In that case, treat it further with more boiling ethyl alcohol until it is free from active matter and the alcohol insoluble portion is no longer sticky.

where
m1 is the mass in g of matter insoluble in alcohol; and m is the mass in g of material taken for the test.
The sample is oxidized by gently heating with sodium nitrate. Silica is removed and the condensed phosphates are hydrolyzed and precipitated as ammonium phosphomolybdate by the addition of anmonium molybdate. The precipitate is washed with dilute potassium nitrate solution and the phosphorus in the washed precipitate is determined by titration with standard sodium hydroxide solution using phenolphthalein as an indicator.
Silica dish, 7-cm diameter
Beaker, 250-ml capacity
Buchner flask, 500-ml capacity with a sintered glass filter funnel
Volumetric flask, 500-ml capacity
Funnel, 7.5 cm diameter
Wide mouthed flat-bottomed flask, 500-ml capacity
Hydrochloric acid: (i) concentrated and (ii) 1:1 dilution
Nitric acid, specific gravity 1.4 g/l
11Ammonium molybdate reagent, dissolve 90 g of ammonium molybdate in hot distilled water. Add 240 g of ammonium nitrate and stir to dissolve. Cool and add 30 ml of concentrated ammonia solution (specific gravity, 0.880). Dilute to 1 litre.
Potassium nitrate, 1.25 % solution in distilled water
Sodium hydroxide, 1.0 M accurately standardized
Sulfuric acid, 1.0 M accurately standardized
Phenolphthalein solution, 1 % solution (m/v) in ethyl alcohol
Accurately weigh about 1.5 of the sample in a silica dish and add a small amount of sodium nitrate. Mix well and heat gently over a Bunsen burner until the sample is completely oxidized. Cool and add 15 ml of concentrated hydrochloric acid and evaporate to dryness. Add further 15 ml of concentrated hydrochloric acid and repeat the evaporation procedure. Finally extract the residue in 25 ml of 1:1 hydrochloric acid and filter through a sintered glass crucible. Wash the crucible with 25 ml of dilute hydrochloric acid and then wash four times with 50 ml of distilled water. Collect the filtrate and washings and make up to 250 ml in a volumetric flask.
Pipette 50-ml aliquot from the volumetric flask in a 250-ml beaker. Add 10 ml of nitric acid and boil for 15 min. Cool and add 100 ml of distilled water and adjust the temperature of the solution between 40 °C and 45 °C. Add 50 ml of ammonium molybdate solution (previously heated to 40 °C) slowly with constant stirring. Allow to stand for 30 min. Filter the precipitate through a quantitative filter paper and wash with 1.25 % potassium nitrate solution till 5 ml of the filterate with 1 drop of phenolphthalein does not require more than 3 to 5 drops of 0.1 mol caustic potash to produce a pink colour.
Transfer the filter paper with the precipitate to 500-ml wide-mouth flat-bottom flask and add 100 ml of distilled water. Heat over a water-bath for 15 min, cool and titrate with 1 M sodium hydroxide using 1 ml of phenolphathalein, till the pink colour just appears. Add 2 ml in excess. Shake well, heat to 60 °C in a water-bath. Cool and back titrate against 1 M sulfuric acid till the pink colour just disappears. Note the volume of normal sodium hydroxide to react with the precipitate.
Total phosphorus as tripolyphosphate on the original sample, X, %,
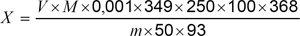
where
V is the volume of sodium hydroxide required to react with the precipitate; M is the molarity of sodium hydroxide, and m is the mass of sample taken for test.

where
X is the total phosphorus as tripolyphosphate on the original sample %; and Y is the matter insoluble in alcohol %.
pH determination should be made in an acid-free atmosphere.
pH meter, any standard electrometric instrument, equipped with a low sodium error glass electrode. The instrument shall be calibrated and standardized with standard buffer solutions (see B.8.3.2) before use.
Volumetric flask, 1 000-ml capacity
Distilled water, shall be boiled thoroughly or purged with carbon dioxide-free air to remove carbon dioxide, and shall be protected with soda lime or soda asbestos while cooling and in storage. The pH of this water shall be between 6.2 and 7.2 at 30 °C.
The residue on evaporation when heated at 105 °C for 1 h shall not exceed 0.5 mg per litre.
Standard buffer solution, any two suitable buffer solutions within the pH range of 9 to 11 at 30 °C for calibrating the pH meter.
Weigh 10 g ± 0.001 g of the material and transfer to a 1-litre volumetric flask. Partially fill the flask with distilled water and agitate until the sample is completely dissolved Adjust the temperature of the solution and the distilled water to 30 °C ± 0.5 °C. and fill to the calibration mark with distilled water. Stopper the flask, mix thoroughly, and allow the solution to stand at a temperature of 30 °C for 2 h prior to measuring the pH. Measure the pH of the solution using a glass electrode.
The term non-detergent organic matter includes hydrocarbons, fatty alcohols and perfumes. Using petroleum ether and under the conditions prescribed, non-detergent organic matter only is extracted
13leaving any alkylolamide present in the material.
Separating funnels, 1 000-ml capacity.
Wide mouthed flat-bottomed flask, 200-ml capacity
Buchner flask, 500-ml capacity, fitted with a sintered glass filter funnel (porosity 4)
Ethyl alcohol, 50 %, 70 %. 90 % and 96% (by volume)
Petroleum ether, boiling range 40 °C to 60 °C non-volatile residue at 80 °C maximum 0.001%
Acetone, non-volatile residue at 80 °C maximum 0.001 %
Removal of inorganic salts — Weigh accurately about 5 g of the material in a 150-ml squat beaker. Extract with 50 ml of hot 90 % ethanol by heating on the steam bath for about 2 min stirring and breaking up any hard lumps with a glass rod flattened at the end.
Allow the solid matter to settle and decant the hot alcoholic solution through a sintered glass filter funnel (porosity 4) fitted to a 500-ml Buchner flask to which suction is applied. Repeat the extraction in a similar manner with five further consecutive 30-ml quantities of boiling 90 % ethanol. Pass each extract in turn through the filter into the flask.
Transfer quantitatively all the combined filtrate from the Buchner flask to a 1-litre separating funnel and rinse the flask four times with 40-ml quantities of distilled water, transferring each wash in turn to the separating funnel. Add 100 ml of petroleum ether, swirl gently to ensure adequate mixing and allow the two phases to separate. Run off the aqueous alcoholic layer into a second separating funnel, and extract with 75 ml of petroleum ether. Repeat the extraction of the aqueous alcoholic phase in the third separating funnel with a further 75 ml of petroleum ether. Combine the three ether extracts in the first separating funnel. Rinse each of the two empty funnels with a few millilitres petroleum ether and add the rinsing to the combined ether extracts.
Wash the combined ether extracts and rinsing (see B.9 4.2) with four successive 50-ml portions of 70 % ethyl alcohol, shaking and removing the alcoholic phase each time. Transfer the ether layer in stages to a tared flask and evaporate off the solvent. Add 10 ml of acetone and evaporate off the solvent. Rotate the flask on a steam bath during the operation. Cool the flask to about 60 °C to 65 °C, gently blow out the last traces of solvent with a current of dry air, cool in a desiccator and weigh.

where
m1 is the mass g of the non-detergent organic matter in the flask; and m is the mass in g of the material taken for the test.
Starting with a fresh portion of the material, proceed as described under B.6.4 but do not dry or weigh the matter insoluble in alcohol. After filtering and washing the residue thoroughly with hot ethyl alcohol, change the receiver, extract the residue with successive portions of distilled water at about 60 °C, and wash the residue several times to remove all the water solubles. Dry the sintered glass funnel with the residue in an air-oven at a temperature of 105 °C ± 2 °C until a constant mass is obtained

where
m1 is the mass in g of matter insoluble in water; and m is mass in g of material taken for the test.
The method is based on hydrolysis and dehydration of the carboxymethylcellulose to furfural derivatives, which produce a green colour with a solution of anthrone in 60 % sulfuric acid. The method is not specific for carboxymethylcellulose, as most celulose derivatives, and also other carbohydrates such as sucrose, react similarly. The intensity of the colour depends upon the degree of substitution of the carboxymethylcellulose, being less intense with higher degrees, and it is essential to prepare a standardization graph from similar material to that in the sample, or to express the result in terms of an arbitrary standard.
The reagents shall be of a recognized analytical reagent quality. Distilled water or water of at least equal purity shall be used.
15Sulfuric acid, concentrated, specific gravity, 1,84 g/cm3
Sulfuric acid, dilute, add 60 ml of the concentrated sulfuric acid slowly to 40 ml of water, cooling well dilute to 100 ml with water when cold.
Anthrone solution, dissolve 0.2 g of anthrone in about 50 ml of the concentrated sulfuric acid, which has been added to 5 ml of water. Cool and dilute to 200 ml with the concentrated sulfuric acid. Allow the solution to stand for 4 h before use. Do not use a solution that is more than 24 h old.
Before use, dilute 60 ml of the above solution to 100 ml, adding the strong solution to the requisite amount of water, cooling well and diluting to volume with water when cold.
One-mark volumetric flask, of capacity 50-ml, 100-ml, and 200-ml.
Filter, sintered glass, porosity No. 4
Spectrophotometer, set to read at 650 nm for absorptiometer with suitable filter and 1 cm glass cell.
Dissolve 1 g of the surface-active agent in the diluted sulfuric acid and dilute to 100 ml with this acid. If persalts are present, heat the weighed sample for 2 h in an oven at 150 °C, then allow to cool before dissolving in the acid. Filter the solution through the sintered glass filter, using a little Kieselguhr to aid filtration. Mix 5 ml of the filtered solution in a 50 ml one-mark volumetric flask with 30 ml of the anthrone solution. Heat the mixture in a boiling water bath for 15 minutes. Cool and dilute to the mark with the diluted sulfuric acid. Measure the optical density of the solution in a 1 cm cell at 625 mµ against a blank prepared from 30 ml of the reagent diluted to 50 ml with the diluted sulfuric acid.
Prepare a standardizing graph from figures obtained by treating known amounts of carboxymethylcellulose by the above procedure.
Calculate the percentage of carboxymethylcellulose in the sample by reference to the graph.
16(normative)
In drawing preparing, storing and handling samples, the following precautions shall be observed.
Samples shall be taken in a protected place, not exposed to damp air, dust or soot.
The sampling instruments shall be clean and dry when used.
The samples, the material being sampled, the sampling instruments and the containers for samples shall be protected from adventitious contamination.
The samples shall be placed in clean and dry glass containers. The sample containers shall be of such a size that they are almost completely filled by the sample.
Each container shall be sealed airtight after filling, and marked with full details of sampling, date of sampling, batch or code number, name of manufacturer, and other important particulars of the consignment.
The samples shall be stored in such a manner that the temperature of the material does not vary unduly from the normal temperature, and that they are protected from light.
Lot — In a single consignment, all the packages containing non-soapy detergents of the same type and form, and drawn from the same batch of manufacture, shall constitute a lot. If the consignment consists of packages containing non-soapy detergents of different types and forms, then the packages containing non-soapy detergents of the same type form and batch of manufacture shall be grouped together, and such group shall constitute a separate lot.
For ascertaining the conformity of the lot to the requirements prescribed in this standard, tests shall be carried out on each lot separately. The number (n) of packages to be selected for drawing the samples shall depend upon the size (N) of the lot and shall be in accordance with Table 2.
17| (Clause C.2.2) No. of packages in the lot (N) |
No. of packages to be selected (n) |
|---|---|
| 4 to 15 16 to 40 41 to 65 66 to 110 111 and above |
3 4 5 7 10 |
| NOTE When the size of the lot is 3 packages or less, the number of containers to be selected and the criteria for judging the conformity of the lot to the specifications should be as agreed on between the purchaser and the supplier. | |
The packages shall be selected at random and to ensure randomness of selection, a random number table shall be used. In case such tables are not available, the procedure given in C.2.3.1 may be adopted.
Starting from any package, count all the packages in one order as 1, 2, 3,…, up to r and so on, where r is the integral part of N/n, (N being the lot size and n the number of packages to be selected). Every rth package thus counted shall be withdrawn to give a sample for the purposes of test.
Powders — From each one of the packages selected as in C.2, draw at random one or more containers. The material in the containers so chosen shall be nearly thrice the quantity required for purpose of test as indicated in C.4.
The material from the containers selected as in C.3.1.1 shall be disintegrated, if necessary, and mixed thoroughly to give the gross sample for the package.
Pastes — From each one of the packages selected as in C.2.2, draw at random one or more containers. The material in the containers so chosen shall be nearly thrice the quantity required for purposes of test as indicated in C.4.
The material from the containers selected in C.3.1.2 shall be mixed by thoroughly to give the gross sample for the package.
Segregate carefully the gross samples (see C.3.1.1 and C.3.1.2) of powders, and pastes. From the gross representing each form of synthetic detergent take a small but equal quantity of material and mix thoroughly into a composite sample which should be of a size sufficient to carry out
18triplicate testing for all the characteristics specified under C.4. The composite samples representing each form and type of synthetic detergent shall be divided into three equal parts, one for the purchaser, another for the supplier, and the third for the referee.
The remaining portion of the material in each of the gross samples shall be divided into three equal pans, each forming an individual sample. One set of individual samples, representing the n selected packages shall be for the purchaser, another for the supplier, and the third for the referee.
All the composite and individual samples shall be transferred to separate containers. These containers shall then be sealed airtight with stoppers, and labelled with full particulars of identification given in C.1.5.
The referee samples shall consist of a composite sample and a set of individual samples. All the containers shall bear the seals of both the purchaser and the supplier, and shall be kept at a place agreed to between the two parties.
Referee samples shall be used in case of any dispute between the purchaser and the supplier.
Tests for the determination of active ingredient shall be performed on each of the individual samples.
Tests for the determination of the remaining characteristics specified in Table 1 shall be conducted on the composite sample.
For the characteristic, which has been determined on the individual sample, the mean (X) and the range (R) of test results shall be calculated as follows:
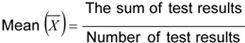
Range (R) The difference between the maximum and the minimum value of test results.
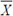 - KR) shall be calculated from the relevant test results [see also C.5.1 (d)]. If the value so obtained is greater than or equal to the minimum limit, the lot shall be declared as conforming to the requirement for the characteristic. 19
- KR) shall be calculated from the relevant test results [see also C.5.1 (d)]. If the value so obtained is greater than or equal to the minimum limit, the lot shall be declared as conforming to the requirement for the characteristic. 19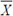 + KR) shall be calculated from the relevant test results [see also C.5.1 (d)]. If the value so obtained is less than or equal to the maximum limit, the lot shall be declared as conforming to the requirement for the characteristic.
+ KR) shall be calculated from the relevant test results [see also C.5.1 (d)]. If the value so obtained is less than or equal to the maximum limit, the lot shall be declared as conforming to the requirement for the characteristic.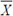 ± KR) shall be calculated from the relevant test results [see also C.5.1 (d)]. If the value so obtained lies between the two specification limits, the lot shall be declared as conforming to the requirement for the characteristic.
± KR) shall be calculated from the relevant test results [see also C.5.1 (d)]. If the value so obtained lies between the two specification limits, the lot shall be declared as conforming to the requirement for the characteristic.| Clause C.5.1 (d) Acceptable quality level |
Value of ‘K’ |
|---|---|
| Not more than 3.0 % defectives Not more than 1.5 % defectives Not more than 0.5 % defectives |
0.4 0.5 0.6 |
For declaring the conformity of the lot to the requirements of all the remaining characteristics determined on the composite sample, the test results for each one of the characteristics shall satisfy the relevant requirement given in Table 1 of this standard.
20IS: 4955: 1993, Specification for household synthetic detergents, published by the Indian Standards Institution.
21