
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.

EAS 105: 1999
ICS 67.140
EAST AFRICAN COMMUNITY
© EAS 1999
First Edition 1999
i| Page | ||
| Foreword | iv | |
| 1 | Scope | 1 |
| 2 | Normative references | 1 |
| 3 | Terms and definitions | 1 |
| 4 | Requirements | 3 |
| 4.1 | Description | 3 |
| 4.2 | Freedom from extraneous matter and impurities | 3 |
| 4.3 | Cup-test | 3 |
| 4.4 | Particle size (Grind) | 3 |
| 4.5 | Hygienic conditions: | 4 |
| 4.6 | Other Requirements | 4 |
| 5 | Packing, marking and labelling | 4 |
| 5.1 | Packing | 4 |
| 5.2 | Size of packages | 5 |
| 5.3 | Marking and labelling | 5 |
| 6 | Sampling | 5 |
| 7 | Tests | 5 |
| ANNEX A (normative) | 6 | |
| A.1 | Sampling of roasted and ground coffee | 6 |
| A.2 | Scale of sampling | 6 |
| A.3 | Test samples and reference samples | 7 |
| A.4 | Number of tests and criteria for conformity | 8 |
| ANNEX B (normative) Determination of colour and roast | 9 | |
| B.1 | Apparatus | 9 |
| B.2 | Procedure | 9 |
| ANNEX C (normative) Cup-test | 10 | |
| C.1 | Procedure | 10 |
| C.2 | Evaluation of the liquor | 10 |
| ANNEX D (normative) Determination of particle size (grind) | 12 | |
| D.1 | Apparatus | 12 |
| D.2 | Procedure | 12 |
| ANNEX E (normative) Determination of moisture | 14 | |
| E.1 | Procedure | 14 |
| E.2 | Calculation | 14 |
| ANNEX F (normative) Determination of total ash | 15 | |
| F.1 | Procedure | 15 |
| F.2 | Calculation | 15 |
| ANNEX G (informative) Determination of acid insoluble ash | 16 | |
| G.1 | Reagent | 16 |
| G.2 | Procedure | 16 ii |
| G.3 | Calculation | 16 |
| ANNEX H (normative) Determination of water insoluble ash | 17 | |
| H.1 | Procedure | 17 |
| H.2 | Calculation | 17 |
| ANNEX J (normative) Determination of alkalinity of soluble ash | 19 | |
| J.1 | Reagents | 19 |
| J.2 | Procedure | 19 |
| ANNEX K (normative) Determination of water-soluble matter | 20 | |
| K.1 | Procedure | 20 |
| K.2 | Calculation | 20 |
| ANNEX L (normative) Determination of caffeine content | 21 | |
| L.1 | Reagents | 21 |
| L.2 | Procedure | 21 |
| L.3 | Calculation | 22 |
| ANNEX M (normative) Determination of petroleum ether extract | 23 | |
| M.1 | Apparatus | 23 |
| M.2 | Reagents | 23 |
| M.3 | Procedure | 23 |
| M.4 | Calculation | 23 |
Development of the East African Standards has been necessitated by the need for harmonizing requirements governing quality of products and services in the East African Community. It is envisaged that through harmonized standardization, trade barriers that are encountered when goods and services are exchanged within the Community will be removed.
In order to achieve this objective, the Community established an East African Standards Committee mandated to develop and issue East African Standards.
The Committee is composed of representatives of the National Standards Bodies in Partner States, together with the representatives from the private sectors and consumer organizations. Draft East African Standards are circulated to stakeholders through the National Standards Bodies in the Partner States. The comments received are discussed and incorporated before finalization of standards, in accordance with the procedures of the Community.
East African Standards are subject to review, to keep pace with technological advances. Users of the East African Standards are therefore expected to ensure that they always have the latest versions of the standards they are implementing.
© East African Community 1999 – All rights reserved
East African Community
AICC Building
Kilimanjaro Wing, 5th Floor
P.O. Box 1096
Arusha
Tanzania
Tel: 255 27 2504253/8
Fax: 255 27 2504255
E-mail: eac@eachq.org
Web: www.eachq.org
ISBN
Roasted and ground coffee for the preparation of a cup of coffee is highly prized for its relatively full aroma.
This standard has been prepared in order to ensure the quality of roasted and ground coffee and to safeguard the health of consumers.
In the preparation of this standard assistance was derived from IS 3077: 1972, Specification for roasted and ground coffee published by the Indian Standards Institution.
In reporting the result of a test or analysis made in accordance with this standard, if the final value observed or calculated, is to be rounded off, it shall be done in accordance with EAS 124:1999 (see Clause 2).
vRoasted coffee beans and roasted ground coffee – Specification
This East African Standard prescribes the requirements and methods of sampling and test for roasted coffee beans and roasted ground coffee.
The following referenced documents are indispensable for the application of this document. For dated references, only the edition cited applies. For undated references, the latest edition of the referenced document (including any amendments) applies.
EAS 124:1999, Rounding off numerical values
EAS 123:1999, Distilled Water – Specification
TZS 109:1981, Code of hygiene for food processing units – General
EAS 106:2000, Coffee and its products – Vocabulary
TZS 419:1989, Coffee – Determination of caffeine content (reference method).
For the purposes of this standard, the following terms and definitions shall apply.
the organoleptic attribute producing a sharp and pleasing flavour, particularly strong with coffee of certain origins; as opposed to a caustic overfermented sour or bitter flavour.
odour characteristic of roasted and ground coffee with a pleasant sensation.
a taste sensation or mouth feeling of more viscocity usually associated with a strong, full, pleasant coffee flavour, as opposed to being thin, but in no way reflecting any increase in true physical viscosity of the fluid.
foreign flavour originating from contaminants, for example, diesel oil, cardamom etc. present in coffee.
1a dark coloured roasted and ground coffee, whose darkly roasted beans have visible oil having run out of their ruptured cells to the bean surfaces. A reason for a dark roast, beyond full flavour development, is to drive off objectionable flavours.
an undersirable test and odour after storage with damage coffees, resembling the odour of freshly uncovered earth, usually due to moulds.
complex combination of the olfactory and gustatory attributes perceived during testing, which may be influenced by tactile and thermal effects.
a greenish grassy, or greenish flavour particularly strong with early pickings of new crop Arabicas which have been picked prematurely.
a taste caused by under-roasting, thus failing to develop full coffee flavour, somewhat pasty. A sourish flavour imparted by “green” beans, for example, those that have never matured. It should be distinguished from grassiness.
a flavour which is often caustic, unpleasantly sharp, rough, or irritating sometimes described as rioy.
a light coloured roasted and ground coffee, which would normally be carried out to below full flavour development, to attain high cup acidity.
a medium coloured roasted and ground coffee, whose beans have been roasted such that they are neither a light roast nor a dark roast, and have attained full flavour and aroma development.
a flavour often due to poor storage, especially with Robustas. Can be due to lack of sufficient drying and aging or overheating. Mustiness due to age is not undesirable.
a fresh light coffee flavour and aroma which enhances the normal characteristics of a coffee blend, particularly flavour and acidity, as distinguished from wildness or greenness which are frequently present in new crop coffees.
a flavour and aroma in which the normal characteristics of a mature, greenish coffee are weakened or toned down, particularly acidity and flavour, but which can also reflect a deterioration of these qualities into a woody or papery flavour with little or no body.
an unpleasant flavour, which produces a penetrating character that cannot be hidden by blending. It is somewhat medicinal (iodine) with possibly woody or fermented overtones.
a sharp excessively acidic biting flavour.
2coffee polluted by stinkers, which renders the coffee unfit for human consumption. Unpalatable coffee or foul coffee.
the presence of one or more extreme flavour characteristics, usually akin to sourishness or fermentation, found in poorly prepared coffees.
a hard woodlike flavour often due to old coffee which has been stored too long as green beans.
The roasted and ground coffee shall be prepared only from green coffee beans, which are properly cleaned and are free from any insect infestation. Such green coffee beans shall be suitably roasted and ground. The roasting may be done to the desired colour, designated as light roast, medium roast and dark roast, when determined by the method prescribed in Annex B.
The roasted and ground coffee shall be free from any artificial colouring, flavouring or extraneous matter and shall be free from rancid or off-flavour.
The roasted and ground coffee shall be evaluated for cup-test in accordance with the procedure prescribed in Annex C.
The roasted and ground coffee shall be graded as extra fine, fine, medium and coarse in accordance with Table 1.
The particle size shall be determined by the method prescribed in Annex D.
| Grade | Percent by mass retained on 710-micron sieve, max |
Percent by mass retained on 500-micron sieve, max |
Percent by mass passing through 355-micron sieve, max |
|---|---|---|---|
| Extra fine Fine Medium Coarse |
5 10 20 30 |
10 15 20 25 |
Above 50 50 30 15 |
The roasted and ground coffee shall be manufactured in premises built and maintained under hygienic conditions as prescribed in TZS 109:1981 (see Clause 2). The handling equipment like roasters, grinders, and packing equipment shall be clean and free from any objectionable odour.
The roasted and ground coffee shall comply with the requirements given in Table 2.
| Characteristics | Requirements | Method of Test (refer to annexes) |
|---|---|---|
| Moisture (at time of packing), percent by mass, max. |
3,0 to 5,4 | E |
| Total ash (on dry basis), percent by mass. |
3,0 to 6,0 | F |
| Acid insoluble ash (on dry basis) percent by mass, max. |
1,0 | G |
| Water soluble ash (on dry basis) percent by mass of total ash, min |
65,0 | H |
| Alkalinity of soluble ash in ml of 0,1 moles/hydrochloric acid, (on dry basis) per gram of material |
3,5 to 5,0 | J |
| Water soluble matter (on dry basis), percent by mass |
26,0 to 35,0 | K |
| Caffeine (on dry basis), percent by mass, min |
1,0 | L |
| Petroleum ether extract (on dry basis), percent by mass, min |
8,5 | M |
The roasted and ground coffee shall be packed in clean, sound and suitable food grade containers like tinplate, glass, bags made of cellophane, metal foil, plastic films and laminates. The roasted and ground coffee may also be vacuum packed or packed in inert gas.
The package shall be temper-proof.
4The quantity of roasted and ground coffee when packed in rigid containers of glass, plastic or metal shall be 50 g. 100 g, 250 g, 1 kg and thereafter by steps of 1 kg.
The quantity when packed in containers other than rigid containers of glass, plastic or metal shall be 50 g. 100 g, 500 g (with tolerance of ± 0,5 g) and thereafter by steps of 1 kg (with tolerance of ± 0,75 g).
The following particulars shall be marked legibly and indelibly on the label of the container:
Each pack and container may also be marked with the Certification Mark for the respective country’s Bureau of standards.
| NOTE | Details of conditions under which a licence for the use of a Certification Mark may be granted to manufacturers, may be obtained from the respective Bureau of standards. |
The method of drawing representative samples of the material and the criteria for conformity shall be as prescribed in Annex A, except moisture, for which a sample shall be taken from each batch before packing and sealing suitably.
Tests shall be carried out by the appropriate methods referred to in 4.1, 4.3, 4.4, 4.5 and those referred to in Table 2.
For the quality of reagents, unless specified otherwise, pure chemicals shall be employed in tests and distilled water in accordance with EAS 123:1999 (see Clause 2) shall be used wherever the use of water as a reagent is intended.
| NOTE | ‘Pure chemicals’ should mean chemicals that do not contain impurities, which affect the results of analysis. |
(normative)
In drawing, preparing, storing and handling samples, the directions and precautions given in A.1.1 to A.1.6 shall be observed.
Samples shall be taken in a protected place not exposed to damp air, dust or soot.
The sampling instrument, preferably a spoon or spatula shall be clean and dry when used.
The samples, the material being sampled, the sampling instrument and the containers for samples, shall be protected from adventitious contamination.
The samples shall be replaced in clean and dry glass or tin containers. The sample containers shall be of such size that they are almost completely filled by the sample.
Each container shall be sealed airtight after filling and marked with full details of sampling batch or code number, name of the manufacturer and other important particulars of the consignment and lot.
Samples shall be stored in such a manner that the temperature of the material does not vary unduly from the normal temperature and that they are protected from light.
All the containers of the same size in a single consignment of material drawn from a single batch of manufacture shall constitute a lot.
Samples shall be tested for each lot separately for ascertaining conformity of the material to the requirements of this specification. The total number of containers to be selected from the lot shall depend on the size of the lot and shall be in accordance with columns one and two of Tables 3 and 4.
These containers shall be chosen at random from the lot and for this purpose a random number table as agreed to between the purchaser and the vendor shall be used. In case such a table is not available, the following procedure shall be adopted:
Starting from any container, count in one order as 1, 2, etc. up to r ± 0,5 and so on, where r is the integral part of N/n (N being the number of containers in the lot and n the number of containers to be selected). Every rth container so counted shall be withdrawn to constitute the sample.
6The sample container of net contents less than 500 g selected according to A.2.2 and columns one and two of Table 3 shall be equally divided at random into a number of groups specified in column three of table 3. Each sample container of net content 500 g or more selected according to A.2.2 and columns one and two of Table 4 shall be treated as one group.
Preparation of individual samples: The contents of all the containers in a group shall be poured out and mixed thoroughly. About 360 g of the material shall be taken from this and divided into three equal parts. Each part, so obtained, shall be transferred to a sample container, which shall be sealed airtight and labelled with the particulars given in A.1.5. The sample so obtained shall be divided into three sets in such a way that each set has a sample representing each group. One of these sets shall be marked for the purchaser, another for the vendor and the third for the referee.
| Number of containers in the lot, N |
Total number of containers to be Selected, n |
Number of groups into which sample container have to be divided |
|---|---|---|
| Up to 50 | 9 | 1 |
| 51 to 300 | 18 | 2 |
| 301 to 500 | 20 | 2 |
| 501 to 1000 | 30 | 3 |
| 1001 to 3000 | 40 | 4 |
| 3001 and over | 50 | 5 |
| Number of containers in the lot, N |
Total number of container to be selected, n |
|---|---|
| Up to 50 | 2 |
| 51 to 300 | 3 |
| 301 to 500 | 4 |
| 501 to 1000 | 5 |
| 1001 and over | 6 |
Preparation of a composite sample: From the mixed material of each group remaining after taking the sample in A.3.2, equal quantities of the material shall be taken and mixed up together so as to form a composite sample representing the lot as a whole and weighing not less than 90 g. This composite sample shall be divided into three equal parts and transferred to clean, dry sample containers and labelled with all particulars given in A.1.5. One of these composite samples representing the lot as a whole shall be for the purchaser, another for the vendor and the third for the referee.
Referee samples: Referee samples for a lot shall consist of a set of samples obtained in A.3.2 and composite sample obtained in A.3.3, marked for this purpose, and shall bear the seals of the purchaser and the vendor. These shall be kept at a place and under conditions agreed to between the purchaser and the vendor.
The tests for the visual characteristics, particle size, water soluble matter and evaluation for cup-test shall be conducted individually on each of the samples in a set as obtained in A.3.2 representing all the groups of sample containers from the lot.
The lot shall be considered as conforming to the requirements of visual characteristics, particle size, water soluble matter and evaluation for cup-test, if every sample in the set passed all the tests mentioned in A.4.1.
The tests for determination of characteristics other than moisture and those covered in 4.1, shall be conducted on the composite sample as obtained in A.3.3. The test for moisture shall be made for each batch on the sample meant for this purpose (see 4.1).
The lot shall be considered as conforming to the requirements regarding the characteristics mentioned in A.4.2, if the composite sample passes all the tests, and the moisture sample satisfies the requirements for moisture.
The lot shall be declared to have conformed to the requirements of this specification if it has been found to be in conformity with the stated requirements in A.4.1 and A.4.2.
8(normative)
Photo-electric reflection meter, of suitable type with search unit 610 Y and tristimulus green filter.
Measure the colour (Y-value) of the roasted and ground coffee by taking the reading in the photoelectric reflection meter using tristimulus green filter. The percent reflectance is recorded as Y-value.
The material shall be graded in the following manner:
| Y -value reading | Grading |
| 6,1 to 8,0 | Light roast |
| 5,0 to 6,0 | Medium roast |
| 3,5 to 4,9 | Dark roast |
(normative)
Note the colour, appearance and aroma of the material.
For the cup-test, take 10 g of sample in a 250-ml cup and add 200 ml of water just brought to the boil. Mix well and allow it to brew for 6 min. Study the acidity, body, and flavour of the liquor by taste.
Serve the coffee in porcelain or glass containers in at least 50 ml portions at a temperature of 60 °C ± 2 °C.
Evaluate the cup quality as per the details given below in the scorecard. If more than one sample is required to be evaluated at one time, the scorecard may be modified.
For defects, deduct 1, 2 or 3 marks depending upon the classification of the defect under suspicion, slight or pronounced.
Evaluation is on the basis of the net score, and the final evaluation shall be under the following categories.
| Fine | Good | Fair | Failing of | Poor |
| 16 - 20 | 12 - 15 | 9 - 11 | 7 - 8 | 0 - 6 |
The roasted and ground coffee shall be deemed to have passed the test, if the net score is above 11.
10
(normative)
Pan, fitting to the sieves.
Shaking machines, of suitable type, adjusted for 28 shakes to 30 shakes per minute.
Make a representative sample by mixing thoroughly 100 g of the sample. Weigh the pan and the 710-micron, 500-micron and 3S5-micron sieves individually and record the mass. S∼ the proper order and pour the sample into the top screen and then close. Fix the unit, to a shaker and shake the sample for 5 min. After 5 min of shaking reweigh the sieves and the;-. experiment once again in the same manner. Report the data as follows:
12| Trial 1 | Trial 2 | |
| Mass in g of the sample taken | 100 | 100 |
| Mass in 710 - micron sieve and coffee | a | m |
| Mass in g of sieve alone | b | n |
| Mass in g of coffee | a - b | m - n |
| Mass in g of 500-micron and coffee | c | p |
| Mass in g of sieve alone | d | q |
| Mass in g of coffee | c - d | p - q |
| Mass in g of 355-micron sieve and coffee | e | r |
| Mass in g of sieve alone | f | s |
| Mass in g of coffee | e - f | r - s |
Calculate the average of the two experiments as gram of coffee retained on 710-micron, 500-micron and 355-micron sieves and grade as indicated in Table1.
13(normative)
Weigh accurately about 5 g of the material in a tared dish (about 8,5 cm in diameter). Place the dish in an oven and dry at 100 °C ± 2 °C for 6 h. Cool the dish in a desiccator and weigh. Dry again at 100 °C ± 2 °C for 30 min, cool in the desiccator and weigh. Repeat the process of heating for 30 min, cooling in a desiccator and weighing until the difference between two successive masses is less than one milligram. Record the lowest mass.

where
14
W1 is the mass in g of the dish with the material before drying W2 is the mass in g of the dish with the material after drying, and m is the mass in g of sample taken.
(normative)
Weigh accurately about 5 g of the material in a porcelain dish. Heat at 100 °C ± 2 °C until water is expelled then heat slowly over a flame until swelling ceases. Ignite in a muffle furnace at 550 °C ± 10 °C until grey ash results. Heat the dish again at 550 °C ± 10 °C for 30 min. Cool the dish in a desiccator and weigh. Repeat this process of heating for 30 min, cooling in a desiccator and weighing, until the difference between two successive weighings is less than one milligram. Record the lowest mass.
| NOTE | The dish containing this ash for the determination of acid insoluble ash (see G.2.1) should be preserved. |
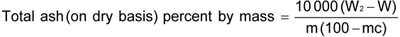
where
15
W2 is the mass in g of the dish with the ash; W is the mass in g of the empty dish; mc is the percentage of moisture as determined in Annex E; and m is the weight in of sample through
(informative)
Dilute hydrochloric acid, approximately 5 mol/I, prepared from concentrated hydrochloric acid.
To the ash contained in the dish (F.1.1), add 25 ml of dilute hydrochloric acid, cover the dish with a watchglass and heat it on a water-bath for 10 min. Allow to cool and filter the contents of the dish through Whatman filter paper No. 42 or its equivalent. Wash the filter paper till the washings are free from the acid. Return the filter paper and the residue to the dish. Keep it in an electric air-oven maintained at 135 °C ± 2 °C for about 3 h. Ignite in a muffle furnace 135 °C ± 10 °C for 1 h. Cool the dish in a desiccator and weigh. Repeat the process of igniting in a mL"le furnace cooling and weighing at half-hour intervals until the difference in mass between two successive weighings is less than one milligram. Record the lowest mass.
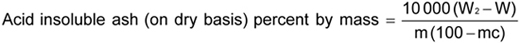
where
16
W2 is the mass in g of the dish with the acid insoluble ash; W is the mass in g of the empty dish (F.2.1); mc is the percentage of moisture as determined in Annex E; and m is the mass in grams of sample taken.
(normative)
Proceed as in Annex F to obtain the total ash. Add 25 ml of water to the ash, stir well, boil for a minute and then filter through Whatman filter paper No. 42 or its its equivalent. Collect the filtrate in a 150-ml beaker, wash the filter paper 4 to 5 times with hot water and collect the washings in the same beaker. Preserve the combined filtrates for estimation of alkalinity of soluble ash (see J.2.1).
Dry the filter paper containing the residue in an oven and ignite it carefully in a weighed platinum or other suitable dish. Complete the ashing in a muffle furnace at 550 °C ± 10 °C for 1 h, cool in a desiccator and weigh. Repeat this process till the difference between two consecutive weighings is less than one milligram. Record the lowest mass.
Water insoluble ash (on dry basis)
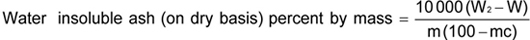
where
W2 is the mass in g of the dish with water insoluble ash; W is the mass in g of the empty dish; mc is the percentage of moisture; and m is the mass, in grams, of sample taken.
Water soluble ash, percent by mass
Water soluble ash, percent by mass = A − B
where
17
A is the total ash percent by mass (F.2.1); and B is the water insoluble ash, percent by mass.
Water soluble ash of total ash
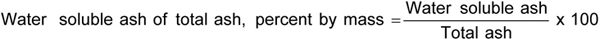
(normative)
Standard hydrochloric acid, 0,1 mol/l
Methyl orange Indicator, dissolve 0,5 g of methyl orange In 500 ml of distilled water. Filter, if, necessary
Titrate the filtrate obtained (H.1.1) with standard hydrochloric acid using the methyl orange indicator. Note the volume in millilitres of the acid used.
Calculate the quantity of 0,1 mol/l hydrochloric acid required to neutralize the water-soluble ash from one gram of the dry material.
19(normative)
Weigh accurately about 2 g of the material in a 500-ml Erlenmeyer flask. and ado 200 ml of water. Reflux over a low flame for 1 h. Cool and filter through a Whatman filter Paper No 1 or its equivalent. Wash three times with 10 ml to 15 ml of water and finally make up to 250 ml in a volumetric: flask. Shake well and pipette a 50-ml aliquot in a tared dish and evaporate on a water bath. After complete evaporation dry for 1 h in an oven at 100 °C ± 2 °C, cool in a desiccator and weigh. Dry again at 100 °C ± 2 °C for 30 min, cool in a desiccator and weigh. Repeat this process of heating for 30 min, cooling in a desiccator and weighing until the loss in mass between the successive weighing is less than one milligram Record the lowest mass
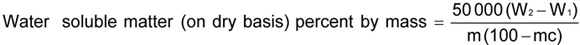
where
20
W2 is the mass in g of the dish with dried water insoluble matter; W1 is the mass in g of the empty dish; mc is the percentage of moisture as determined in Annex E; and m is the calculated mass, in grams, of sample taken for the test.
(normative)
NOTE The method described in TZS 419: 1989 (see Clause 2) shall be carried out for reference purposes only.
Magnesium oxide, powdered.
Dilute sulfuric acid, 1:9 obtained by diluting concentrated sulfuric acid of specific gravity 1.
Chloroform, redistilled.
Potassium hydroxide solution, 1 % (m/v)
Potassium sulfate, crystals, nitrogen-free.
Mercuric oxide, nitrogen-free.
Concentrated sodium hydroxide solution, dissolve about 225 g of sodium hydroxide in 500 mI of water.
Standard sulfuric acid, 0,05 N.
Methyl red Indicator, dissolve 1 g of methyl red in 200 ml of rectified spirit (95 % by volume).
Standard sodium hydroxide solution 0,1 mol/I.
Weigh accurately about 5 g of the material, transfer to a 250-ml Erlenmeyer flask, and add 3 g of magnesium oxide and 100 ml of distilled water. Weigh the flask with contents and boil under a reflux condenser for 45 min, shaking occasionally. Cool and weigh the flask again and add water till the original mass is obtained. Mix well and filter through a dry filter paper directly into a 50-ml graduated flask until exactly 50 ml of the solution (equivalent to half the quantity of the material taken for the test) is obtained. Transfer the solution to a 125-ml separator. Wash the graduated flask with 2 ml of water and add the washings to the separator. Add 4 ml of dilute sulfuric acid. Extract with five 10 ml portions of chloroform, shaking vigorously for 1 min for each extraction. Let the emulsion break; then drain the chloroform into a 125 ml separator. Add 5 ml of potassium hydroxide solution. Shake vigorously for one minute. Let the emulsion break and drain chloroform through cotton plug into a 100-ml Kjeldahl flask. Extract the potassium hydroxide solution with 5 ml of chloroform and add two the Kjeldahl flask. To the digestion flask add 1,30 g ± 0,50 g of potassium sulfate
21and 40 mg ± 5 mg of mercuric oxide. Rinse down the neck of the flask with 43 ml of chloroform. Place the flask on the digestion rack and concentrate chloroform to about 20 ml.
Distill chloroform. Add 2,0 ml ± 0,1 ml of concentrated sulfuric acid of specific gravity 1,84. Digest for 1 h after the acid begins to boil. Cool, and add the minimum quantity of water to dissolve solids. Cool and place thin film of vaseline on the rim of the flask. Transfer the digest and boiling chips to the distillation apparatus and rinse the flask 5 to 6 times with one to twomillilitre portions of water. Place a 125-ml beaker containing a known quantity of standard sulfuric acid. Add 6 ml of concentrated sodium hydroxide solution carefully through the side to the still so that it does not mix, and assemble the distillation apparatus immediately taking care that the dip tube extends well within the standard sulfuric acid contained in the beaker. Mix the contents of the distillation flask and distill until all ammonia has passed over into the standard sulfuric acid. Shut off the heater immediately detach the flask from the condenser. Rinse the condenser thoroughly with water into the beaker. Wash the dip tube carefully so that all traces of the condensate are transferred to the beaker. When all the washings have drained into the beaker, add two or three drops of methyl red indicator solution and titrate with the standard sodium hydroxide solution.
Carry out a blank determination using all the reagents, in the same quantities but without the material.
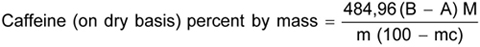
where
| B | is the volume in ml of the standard sodium hydroxide used to neutralize the acid in the blank determination; |
| A | is the volume in ml of the standard sodium hydroxide used to neutralize the acid in the test with the material; |
| M | is the molarity of the standard sodium hydroxide solution; |
| M | is the mass in g of the material in the aliquot; and |
| Mc | is the percentage of moisture as determined in Annex E |
(normative)
Soxhlet extraction apparatus
Petroleum ether, distilled below 60 °C.
Weigh accurately about 10 g of the material in a Suitable thimbleand dry for 2 h at 100 °C ± 2 °C. Place the thimble in the Soxhlet extraction apparatus and extract with the solvent for about, 16 h. Dry the extract contained in the Soxhlet flask, the empty weight of which has been previously determined at 95 °C to 100 °C for an hour. Cool in a desiccator and weigh. Continue the alternate drying and weighing at 30-min intervals until the loss in mass between two successive weighings do not exceed one milligram. Record the lowest mass.
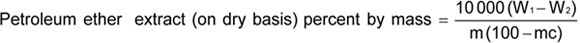
where
23
W1 is the mass in g of the Soxhlet flask with the petroleum ether extract; W1 is the mass in g of the empty Soxhlet flask; mc is the percentage of moisture as determined in Annex E; and m is the mass, in grams, of the material taken for the test.