
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.


CARICOM Regional Organisation for Standards and Quality (CROSQ)
2nd Floor Nicholas House
29 & 30 Broad Street
Bridgetown, St Michael
Barbados
T: 246.622.7670 | F: 246.622.7678
Website: http://www.crosq.org
© CROSQ 2010 – All rights reserved
Unless otherwise specified, no part of this publication may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and microfilm, without permission.
CRCP 4: 2010
CARICOM Regional Organisation for Standards and Quality (CROSQ)
2nd Floor, Nicholas House
29 & 30 Broad Street
Bridgetown, St. Michael
Barbados
T: 246.622.7670 | F: 246.622.7678
Website: http://www.crosq.org
© CROSQ 2010 – All rights reserved. No part of this publication is to be reproduced without the prior written consent of CROSQ.
ISBN 978-976-8234-20-9
ICS 67.120.30
| AMENDMENT NO. | DATE OF ISSUE | TYPE OF AMENDMENT | NO. OF TEXT AFFECTED | TEXT OF AMENDMENT |
|---|---|---|---|---|
This CARICOM Regional Code of Practice was developed under the supervision of the Regional Technical Committee for Foods, Subcommittee for Fish and Fishery Products (hosted by the CARICOM Member State, Jamaica), which at the time comprised the following members:
| Members | Representing |
|---|---|
| Dr. Lloyd Webb (Chairperson) | Caribbean Food and Nutrition Institute |
| Ms. Peta-Ann Hutchinson | Newport Fish and Meats |
| Mr. Garth Wright | Rainforest Seafoods |
| Mrs. Christine Fray-Aiken | University of Technology |
| Ms. Angella Smith | Consumer Affairs Commission |
| Ms. Tanille Latty (Technical Secretary) | Bureau of Standards Jamaica (BSJ) |
| Foreword | 1 | |||
| 1 | Scope | 2 | ||
| 2 | Normative references | 2 | ||
| 3 | Terms and definitions | 3 | ||
| 3.1 | General definitions | 3 | ||
| 3.2 | Aquaculture | 6 | ||
| 3.3 | Live and raw bivalve molluscs | 8 | ||
| 3.4 | Fresh, frozen and minced fish | 9 | ||
| 3.5 | Frozen surimi | 10 | ||
| 3.6 | Quick-frozen coated fish products | 11 | ||
| 3.7 | Salted and dried salted fish | 11 | ||
| 3.8 | Shrimps and prawns | 13 | ||
| 3.9 | Cephalopods | 14 | ||
| 3.10 | Canned fish and shellfish | 14 | ||
| 3.11 | Retail | 15 | ||
| 4 | Pre-requisite programme | 15 | ||
| 4.1 | General | 15 | ||
| 4.2 | Fishing and harvesting vessel design and construction | 15 | ||
| 4.3 | Facility design and construction | 17 | ||
| 4.4 | Design and construction of equipment and utensils | 18 | ||
| 4.5 | Hygiene control programme | 19 | ||
| 4.5.1 | Schedules | 19 | ||
| 4.5.2 | Requirements | 19 | ||
| 4.6 | Personal hygiene and health | 20 | ||
| 4.6.1 | General | 20 | ||
| 4.6.2 | Facilities and equipment | 20 | ||
| 4.6.3 | Personnel hygiene | 21 | ||
| 4.7 | Transportation | 21 | ||
| 4.8 | Product traceability and recall procedures | 21 | ||
| 4.9 | Training | 22 | ||
| 5 | General considerations for the handling of fresh fish, shellfish and other aquatic invertebrates | 22 | ||
| 5.1 | General | 22 | ||
| 5.2 | Time and temperature control | 22 | ||
| 5.2.1 | Minimise deterioration – time control | 23 | ||
| 5.2.2 | Minimise deterioration - temperature control | 23 | ||
| 5.2.3 | Minimise deterioration – handling | 23 | ||
| 6 | Hazard analysis critical control point (HACCP) and defect action point (DAP) analysis | 24 | ||
| 7 | Aquaculture production | 24 | ||
| 7.1 | General | 24 | ||
| 7.2 | General considerations of aquaculture production | 24 | ||
| 7.2.1 | Site selection | 24 | ||
| 7.2.2 | Fish growing water quality | 25 | ||
| 7.2.3 | Source of fry and fingerlings | 25 | ||
| 7.3 | Hazards and defects | 25 | ||
| 7.3.1 | Hazards | 25 | ||
| 7.3.2 | Defects | 25 | ||
| 7.4 | Production operations | 26 v | ||
| 7.4.1 | Feed Supply | 26 | ||
| 7.4.2 | Veterinary drugs | 27 | ||
| 7.4.3 | Growing | 28 | ||
| 7.4.4 | Harvesting | 28 | ||
| 7.4.5 | Holding and transportation | 29 | ||
| 7.4.6 | Storage and transport of live fish | 30 | ||
| 8 | Live and raw bivalve molluscs | 33 | ||
| 8.1 | General | 33 | ||
| 8.2 | Classification and monitoring of growing areas | 33 | ||
| 8.2.1 | Potential hazards and defects | 33 | ||
| 8.2.2 | Classification of growing areas | 34 | ||
| 8.2.3 | Monitoring of growing areas | 34 | ||
| 8.3 | Harvesting and transportation of live bivalve molluscs | 37 | ||
| 8.3.1 | Potential hazards | 37 | ||
| 8.3.2 | Potential defects | 38 | ||
| 8.3.3 | Technical guidance | 38 | ||
| 8.4 | Relaying | 38 | ||
| 8.4.1 | General | 38 | ||
| 8.4.2 | Potential hazards | 39 | ||
| 8.4.3 | Potential defects | 39 | ||
| 8.4.4 | Technical guidance | 39 | ||
| 8.5 | Depuration | 39 | ||
| 8.5.1 | General | 39 | ||
| 8.5.2 | Potential hazards | 40 | ||
| 8.5.3 | Potential defects | 40 | ||
| 8.5.4 | Technical guidance | 40 | ||
| 8.6 | Processing of bivalve molluscs in a distribution centre or an establishment | 41 | ||
| 8.6.1 | General | 41 | ||
| 8.6.2 | Reception | 41 | ||
| 8.6.3 | Conditioning and storage of bivalve molluscs | 42 | ||
| 8.6.4 | Washing, declumping, debyssing and grading | 43 | ||
| 8.6.5 | Packing and labelling | 43 | ||
| 8.6.6 | Storage | 45 | ||
| 8.6.7 | Distribution and transport | 46 | ||
| 8.7 | Processing to reduce or limit target organisms | 47 | ||
| 8.7.1 | General | 47 | ||
| 8.7.2 | Potential hazards | 47 | ||
| 8.7.3 | Potential defects | 47 | ||
| 8.7.4 | Technical guidance | 47 | ||
| 8.8 | Shucking | 48 | ||
| 8.8.1 | Hand and mechanical shucking and washing | 48 | ||
| 8.8.2 | Heat shocking of bivalve molluscs followed by packing | 48 | ||
| 8.9 | Documentation | 49 | ||
| 8.10 | Lot identification and recall procedures | 50 | ||
| 9 | Processing of fresh, frozen and minced fish | 50 | ||
| 9.1 | General | 50 | ||
| 9.2 | Finfish preparation | 50 | ||
| 9.2.1 | General | 50 | ||
| 9.2.2 | Raw, fresh or frozen fish reception (processing step 1) | 50 | ||
| 9.2.3 | Sensory evaluation of fish | 52 | ||
| 9.2.4 | Chilled storage (processing steps 2 and 14) | 52 | ||
| 9.2.5 | Frozen storage (processing steps 3 and 20) | 53 | ||
| 9.2.6 | Control thawing (processing step 4) | 53 | ||
| 9.2.7 | Washing and gutting (processing steps 6 and 7) | 54 | ||
| 9.2.8 | Filleting, skinning, trimming and candling (processing steps 8 and 9) | 55 | ||
| 9.3 | Processing of vacuum or modified atmosphere packed fish | 56 | ||
| 9.3.1 | Weighing (processing step 10) | 56 | ||
| 9.3.2 | Vacuum or modified atmosphere packaging (processing step 11) | 56 vi | ||
| 9.3.3 | Labelling (processing steps 12 and 18) | 57 | ||
| 9.3.4 | Metal detection (processing steps 13 and 19) | 58 | ||
| 9.4 | Processing of frozen fish | 58 | ||
| 9.4.1 | Freezing Pprocess (processing step 15) | 58 | ||
| 9.4.2 | Glazing (processing step 16) | 59 | ||
| 9.5 | Processing of minced fish | 60 | ||
| 9.5.1 | Mincing fish using mechanical separation process (processing step 21) | 60 | ||
| 9.5.2 | Washing of minced fish (processing step 22) | 61 | ||
| 9.5.3 | Blending and application of additives and ingredients to minced fish (processing steps 23 and 24) | 61 | ||
| 9.5.4 | Wrapping and packing (processing steps 17 and 25) | 62 | ||
| 9.6 | Packaging, labels and ingredients | 62 | ||
| 9.6.1 | Reception of packaging, labels and ingredients (processing steps 26 and 28) | 62 | ||
| 9.6.2 | Storage of packaging, labels and ingredients (processing steps 27 and 29) | 63 | ||
| 10 | Processing of frozen surimi | 63 | ||
| 10.1 | General | 63 | ||
| 10.2 | General considerations of hazards and defects for frozen surimi production | 64 | ||
| 10.2.1 | Hazards | 64 | ||
| 10.2.2 | Defects | 64 | ||
| 10.3 | Fish preparation (processing steps 1 to 8) | 64 | ||
| 10.3.1 | General | 64 | ||
| 10.3.2 | Raw, fresh and frozen fish reception (processing step 1) | 64 | ||
| 10.3.3 | Chilled storage (processing step 2) | 65 | ||
| 10.3.4 | Washing and scaling (processing step 6) | 66 | ||
| 10.3.5 | Washing (processing step 8) | 66 | ||
| 10.4 | Fish flesh separation process (processing step 9) | 66 | ||
| 10.4.1 | Potential hazards | 66 | ||
| 10.4.2 | Potential defects | 67 | ||
| 10.4.3 | Technical guidance | 67 | ||
| 10.5 | Washing and de-watering process (processing step 10) | 67 | ||
| 10.5.1 | Potential hazards | 67 | ||
| 10.5.2 | Potential defects | 67 | ||
| 10.5.3 | Technical guidance | 67 | ||
| 10.6 | Refining process (processing step 11) | 68 | ||
| 10.6.1 | Potential hazards | 68 | ||
| 10.6.2 | Potential defects | 68 | ||
| 10.6.3 | Technical guidance | 68 | ||
| 10.7 | Final de-watering process (processing step 12) | 68 | ||
| 10.7.1 | Potential hazards | 68 | ||
| 10.7.2 | Potential defects | 69 | ||
| 10.7.3 | Technical guidance | 69 | ||
| 10.8 | Mixing and addition of adjuvant ingredients process (processing step 13) | 69 | ||
| 10.8.1 | Potential hazards | 69 | ||
| 10.8.2 | Potential defects | 69 | ||
| 10.8.3 | Technical guidance | 69 | ||
| 10.9 | Packaging and weighing (processing step 14) | 70 | ||
| 10.9.1 | Potential hazards | 70 | ||
| 10.9.2 | Potential defects | 70 | ||
| 10.9.3 | Technical guidance | 70 | ||
| 10.10 | Freezing operation (processing step 15) | 71 | ||
| 10.10.1 | Potential hazards | 71 | ||
| 10.10.2 | Potential defects | 71 | ||
| 10.10.3 | Technical guidance | 71 | ||
| 10.11 | Dismantling freezing pan (processing step 16) | 71 | ||
| 10.11.1 | Potential hazards | 71 | ||
| 10.11.2 | Potential defects | 71 | ||
| 10.11.3 | Technical guidance | 71 | ||
| 10.12 | Metal detection (processing step 17) | 71 | ||
| 10.12.1 | Potential hazards | 71 vii | ||
| 10.12.2 | Potential defects | 71 | ||
| 10.12.3 | Technical guidance | 72 | ||
| 10.13 | Boxing and labelling (processing step 18) | 72 | ||
| 10.13.1 | Potential hazards | 72 | ||
| 10.13.2 | Potential defects | 72 | ||
| 10.13.3 | Technical guidance | 72 | ||
| 10.14 | Frozen storage (processing step 19) | 72 | ||
| 10.14.1 | Potential hazards | 72 | ||
| 10.14.2 | Potential defects | 72 | ||
| 10.14.3 | Technical guidance | 72 | ||
| 10.15 | Raw material reception - packaging and ingredients (processing steps 21 and 22) | 73 | ||
| 10.16 | Raw material storage - packaging and ingredients (processing steps 23 and 24) | 73 | ||
| 11 | Processing of quick-frozen coated fish and shellfish products | 73 | ||
| 11.1 | General | 73 | ||
| 11.2 | Identification of hazards and defects | 73 | ||
| 11.2.1 | Potential hazards | 73 | ||
| 11.2.2 | Potential defects | 73 | ||
| 11.3 | Technical guidance | 73 | ||
| 11.4 | Processing operations - fish | 74 | ||
| 11.4.1 | Reception | 74 | ||
| 11.4.2 | Storage of raw material, other ingredients and packaging material | 76 | ||
| 11.4.3 | Frozen fish block or fillet tempering | 77 | ||
| 11.4.4 | Unwrapping, unpacking | 77 | ||
| 11.4.5 | Production of fish core | 78 | ||
| 11.4.6 | Separation of pieces | 79 | ||
| 11.4.7 | Coating | 79 | ||
| 11.4.8 | Pre-frying | 80 | ||
| 11.4.9 | Re-freezing | 81 | ||
| 11.4.10 | Packaging and labelling | 82 | ||
| 11.4.11 | Storage of end products | 82 | ||
| 11.4.12 | Transport of end product | 83 | ||
| 11.5 | Processing operations of molluscan shellfish | 83 | ||
| 11.5.1 | General | 83 | ||
| 11.5.2 | Reception | 84 | ||
| 11.5.3 | Storage of raw material, other ingredients and packaging materials | 85 | ||
| 11.5.4 | Unpacking and unwrapping | 85 | ||
| 11.5.5 | Production of coated molluscan shellfish | 86 | ||
| 11.5.6 | Coating | 87 | ||
| 11.5.7 | Pre-frying | 87 | ||
| 11.5.8 | Re-Freezing – Final freezing | 87 | ||
| 11.5.9 | Packing and labelling | 87 | ||
| 11.5.10 | Storage of end product | 87 | ||
| 11.5.11 | Transport of end product | 87 | ||
| 11.6 | Processing operations of coated shrimp | 87 | ||
| 11.6.1 | General | 87 | ||
| 11.6.2 | Reception | 87 | ||
| 11.6.3 | Storage of raw material, other ingredients and packaging materials | 89 | ||
| 11.6.4 | Unpacking and unwrapping | 89 | ||
| 11.6.5 | Production of coated shrimp | 89 | ||
| 11.6.6 | Coating | 91 | ||
| 11.6.7 | Pre-frying | 92 | ||
| 11.6.8 | Packaging and labelling | 93 | ||
| 11.6.9 | Re-freezing – final freezing | 93 | ||
| 11.6.10 | Casing | 93 | ||
| 11.6.11 | Frozen storage of end product | 94 | ||
| 11.6.12 | Transport of end product | 94 | ||
| 12 | Processing of salted and dried salted fish | 94 | ||
| 12.1 | General | 94 viii | ||
| 12.2 | Preparing for salting | 94 | ||
| 12.2.1 | Splitting, washing and rinsing (processing step 7) | 94 | ||
| 12.2.2 | Filleting, skinning and trimming (processing step 8) | 95 | ||
| 12.2.3 | Round fish (processing step 9) | 95 | ||
| 12.2.4 | Nobbing (processing step 10) | 95 | ||
| 12.2.5 | Gibbing (processing step 11) | 96 | ||
| 12.3 | Salt requirements and salt handling | 96 | ||
| 12.3.1 | Salt requirements (processing step 12) | 96 | ||
| 12.3.2 | Salt handling (processing step 13) | 97 | ||
| 12.4 | Salting and maturing | 98 | ||
| 12.4.1 | General | 98 | ||
| 12.4.2 | Brining (processing step 14) | 98 | ||
| 12.4.3 | Brine injection (processing step 15) | 99 | ||
| 12.4.4 | Wet-salting (processing step 16) | 99 | ||
| 12.4.5 | Dry-salting (processing step 17) | 100 | ||
| 12.4.6 | Pickling (processing step 18) | 100 | ||
| 12.4.7 | Maturing (processing step 19) | 101 | ||
| 12.5 | Sorting, drying, weighing, packaging, wrapping and labelling | 102 | ||
| 12.5.1 | Sorting (processing step 20) | 102 | ||
| 12.5.2 | Drying (processing step 21) | 102 | ||
| 12.5.3 | Weighing, wrapping and packaging (processing step 22) | 103 | ||
| 12.6 | Chill storage (processing step 24) | 103 | ||
| 12.6.1 | Potential hazards | 103 | ||
| 12.6.2 | Potential defects | 103 | ||
| 12.6.3 | Technical guidance | 103 | ||
| 12.7 | Packaging, labels and ingredients (processing steps 25, 26, 27 and 28) | 104 | ||
| 13 | Processing of shrimp and prawns | 104 | ||
| 13.1 | General | 104 | ||
| 13.2 | Frozen shrimp and prawns | 104 | ||
| 13.3 | Shrimp preparation (processing steps 1 to 18) | 104 | ||
| 13.3.1 | Raw fresh and frozen shrimp reception (Process steps) | 104 | ||
| 13.3.2 | Frozen storage | 105 | ||
| 13.3.3 | Controlled thawing | 106 | ||
| 13.3.4 | Chilled storage | 106 | ||
| 13.3.5 | Selection | 107 | ||
| 13.3.6 | Size grading | 107 | ||
| 13.3.7 | Addition of ingredients and use of additives | 107 | ||
| 13.3.8 | Full and partial peeling | 108 | ||
| 13.3.9 | Deveining | 109 | ||
| 13.3.10 | Washing | 109 | ||
| 13.3.11 | Cooking Process | 110 | ||
| 13.3.12 | Peeling of cooked shrimp | 110 | ||
| 13.3.13 | Cooling | 111 | ||
| 13.3.14 | Freezing process | 111 | ||
| 13.3.15 | Glazing | 112 | ||
| 13.3.16 | Weighing, packing and labelling of all products | 112 | ||
| 13.3.17 | Metal detection | 113 | ||
| 13.3.18 | Frozen storage of end product | 113 | ||
| 14 | Processing of cephalopods | 114 | ||
| 14.1 | General | 114 | ||
| 14.2 | Reception of cephalopods (processing step 1) | 114 | ||
| 14.2.1 | Potential hazards | 114 | ||
| 14.2.2 | Potential defects | 115 | ||
| 14.2.3 | Technical guidance | 115 | ||
| 14.3 | Storage of cephalopods | 115 | ||
| 14.3.1 | Chilled storage (processing steps 2 and 10) | 115 | ||
| 14.3.2 | Frozen storage (processing steps 2 and 10) | 116 | ||
| 14.4 | Controlled thawing (processing step 3) | 116 ix | ||
| 14.4.1 | Potential hazards | 116 | ||
| 14.4.2 | Potential defects | 116 | ||
| 14.4.3 | Technical guidance | 116 | ||
| 14.5 | Splitting, gutting and washing (processing steps 4, 5, 6, 11, 12 and 13) | 117 | ||
| 14.5.1 | Potential hazards | 117 | ||
| 14.5.2 | Potential defects | 117 | ||
| 14.5.3 | Technical guidance | 117 | ||
| 14.6 | Skinning and trimming (processing step 7) | 117 | ||
| 14.6.1 | Potential hazards | 117 | ||
| 14.6.2 | Potential defects | 117 | ||
| 14.6.3 | Technical guidance | 118 | ||
| 14.7 | Application of additives | 118 | ||
| 14.7.1 | Potential hazards | 118 | ||
| 14.7.2 | Potential defects | 118 | ||
| 14.7.3 | Technical guidance | 118 | ||
| 14.8 | Grading, packing and labelling (processing steps 8 and 9) | 118 | ||
| 14.8.1 | Potential hazards | 118 | ||
| 14.8.2 | Potential defects | 119 | ||
| 14.8.3 | Technical guidance | 119 | ||
| 14.9 | Freezing (processing step 10) | 119 | ||
| 14.9.1 | Potential hazards | 119 | ||
| 14.9.2 | Potential defects | 119 | ||
| 14.9.3 | Technical guidance | 119 | ||
| 14.10 | Packaging, labels and ingredients – reception and storage | 120 | ||
| 15 | Processing of canned fish, shellfish and other aquatic invertebrates | 120 | ||
| 15.1 | General | 120 | ||
| 15.2 | Technical guidelines | 120 | ||
| 15.3 | General hazards and defects for canned fish, shellfish and other aquatic invertebrates | 121 | ||
| 15.3.1 | Biological hazards | 121 | ||
| 15.3.2 | Chemical hazards | 122 | ||
| 15.3.3 | Physical hazards | 122 | ||
| 15.3.4 | Defects | 122 | ||
| 15.4 | Processing operations | 122 | ||
| 15.4.1 | Reception of raw material | 122 | ||
| 15.4.2 | Storage of raw material, containers, covers and packaging materials | 123 | ||
| 15.4.3 | Unwrapping and unpacking (processing steps 3 and 4) | 124 | ||
| 15.4.4 | Thawing (processing step 5) | 124 | ||
| 15.4.5 | Fish and shellfish preparatory processes (processing step 6) | 125 | ||
| 15.5 | Pre-cooking and other treatments | 126 | ||
| 15.5.1 | General | 126 | ||
| 15.5.2 | Use of brine and other dips | 127 | ||
| 15.5.3 | Packing in containers – filling, sealing and coding (processing step 8) | 128 | ||
| 15.5.4 | Handling of containers after closure (processing step 9) | 131 | ||
| 15.5.5 | Thermal processing (processing step 10) | 131 | ||
| 15.5.6 | Cooling (processing step 11) | 133 | ||
| 15.5.7 | Labelling, casing and storage of finished products (processing steps 12 and 13) | 134 | ||
| 15.5.8 | Transportation of finished products (processing step 14) | 135 | ||
| 16 | Transport | 136 | ||
| 16.1 | General | 136 | ||
| 16.2 | Fresh, refrigerated and frozen products | 136 | ||
| 16.2.1 | Potential hazards | 136 | ||
| 16.2.2 | Potential defects | 136 | ||
| 15.4.1 | Technical guidance | 136 | ||
| 16.3 | Live fish and shellfish | 137 | ||
| 16.4 | Canned fish and shellfish | 137 | ||
| 16.5 | All products | 137 | ||
| 16.5.1 | Technical guidance | 137 | ||
| 17 | Retail | 138 x | ||
| 17.1 | General | 138 | ||
| 17.2 | Reception of fish, shellfish and their products at retail | 138 | ||
| 17.2.1 | Potential hazards | 138 | ||
| 17.2.2 | Potential defects | 138 | ||
| 17.2.3 | Technical guidance | 138 | ||
| 17.3 | Reception of chilled products at retail | 139 | ||
| 17.3.1 | Potential hazards | 139 | ||
| 17.3.2 | Potential defects | 139 | ||
| 17.3.3 | Technical guidance | 139 | ||
| 17.4 | Chilled storage of products at retail | 139 | ||
| 17.4.1 | Potential hazards | 139 | ||
| 17.4.2 | Potential defects | 140 | ||
| 17.4.3 | Technical guidance | 140 | ||
| 17.5 | Frozen storage of products at retail | 140 | ||
| 17.5.1 | Potential hazards | 140 | ||
| 17.5.2 | Potential defects | 140 | ||
| 17.5.3 | Technical guidance | 140 | ||
| 17.6 | Preparation and packaging of chilled seafood at retail | 141 | ||
| 17.6.1 | Potential hazards | 141 | ||
| 17.6.2 | Potential defects | 141 | ||
| 17.6.3 | Technical guidance | 141 | ||
| 17.7 | Preparation and packaging of frozen seafood at retail | 141 | ||
| 17.7.1 | Potential hazards | 141 | ||
| 17.7.2 | Potential defects | 142 | ||
| 17.7.3 | Technical guidance | 142 | ||
| 17.8 | Retail display of chilled seafood | 142 | ||
| 17.8.1 | Potential hazards | 142 | ||
| 17.8.2 | Potential defects | 142 | ||
| 17.8.3 | Technical guidance | 142 | ||
| 17.9 | Retail display of frozen seafood | 143 | ||
| 17.9.1 | Potential hazards | 143 | ||
| 17.9.2 | Potential defects | 143 | ||
| 17.9.3 | Technical guidance | 143 | ||
| Annex A (informative) Potential hazards associated with fresh fish, shellfish and other aquatic invertebrates | 145 | |||
| Annex B (informative) A permanent cleaning and disinfecting schedule | 149 | |||
| Annex C (normative) HACCP and DAP analysis | 150 | |||
| Annex D (informative) Flow Diagrams | 162 | |||
| Annex E (normative) General remarks, addition to the pre-requisite programme | 174 | |||
| Annex F (informative) Examples of unacceptable sensory characteristics | 175 | |||
| Annex G (informative) Optional final product requirements - Salted fish | 176 | |||
| List of tables | ||||
| Table 1 — Fresh white fish sensory evaluation criteria | 52 | |||
| Table C.1 — A product description for canned tuna in salted water | 153 | |||
| Table C.2 — Examples of pre-harvest and harvest hazards in incoming fish and shellfish | 154 | |||
| Table C.3 — Examples of hazards introduced in the post harvest and further processing of fish and shellfish | 155 | |||
| Table C.4 — An example of potential hazards for canned tuna | 155 | |||
| Table C.5 — An example of potential defects of canned tuna | 155 xi | |||
| Table C.6 — An example of the significant hazard survival of C. botulinum at the step of heat processing for canned tuna | 157 | |||
| Table C.7 — A schematic example of a hazard analysis with corresponding control measures and the application of the CODEX decision tree for the determination of a critical control point at processing step 12 of the example process as set out in Figure D.1 | 158 | |||
| Table C.8 — A schematic example of a defect analysis with corresponding control measures and the application of the CODEX decision tree for the determination of a defect action point at processing step 2 of the example process as set out in Figure D | 158 | |||
| Table C.9 — An example of the results of the application of HACCP principles to the two specific steps in the canned tuna process (tables 8 and 9), for a CCP and a DAP, respectively | 160 | |||
| Table F.1 — A product description for canned tuna in salted water | 175 | |||
| Table G.1 — Species used to produce dried salted fish | 176 | |||
| List of figures | ||||
| Figure C.1 — Summary of the implementation of a HACCP and defect analysis | 151 | |||
| Figure D.1 — Example of a flow diagram for a processing line of canned tuna fish in brine | 162 | |||
| Figure D.2 — Example of a flow chart for aquaculture production | 163 | |||
| Figure D.3 — Example of a simplified flow diagram for production of live and raw bivalve molluscs | 164 | |||
| Figure D.4 — Example of a flow chart of a fish fillet preparation line, including MAP, mincing and freezing process | 165 | |||
| Figure D.5 — Example of a flow chart of a frozen surimi production process | 166 | |||
| Figure D.6 — Example of a flow chart for the processing of coated fish products | 167 | |||
| Figure D.7 — Example of a flow chart for coated molluscan shellfish processing | 168 | |||
| Figure D.8 — Example of a flow chart of a coated shrimp processing line | 169 | |||
| Figure D.9 — Example of a flow chart for salted and dried salted fish processing line | 170 | |||
| Figure D.10 — Example of a flow chart of a shrimp and prawn processing line | 171 | |||
| Figure D.11 — Example of a possible squid processing line | 172 | |||
| Figure D.12 — Example of a flow chart for the processing of canned fish and shellfish | 173 | |||
This CARICOM Regional Code of Practice has been prepared through the CARICOM Regional Organisation for Standards and Quality (CROSQ). It is an adaptation of the CODEX Alimentarius Commission Code of Practice for Fish and Fishery Products. This Code of Practice recommends general guidelines on the production, storage and handling of fish and fishery products on board fishing vessels and on shore. It incorporates the Hazard Analysis Critical Control Point (HACCP) approach, which is recommended to ensure the hygienic production of fish and fishery products to meet health and safety requirements.
A pre-requisite programme is described in the Code of Practice covering technological guidelines and the essential requirements of hygiene in the production of fish, shellfish and their products, which are safe for human consumption, and otherwise meets the requirements of the appropriate CODEX Alimentarius Commission product standards.
This Code of Practice will assist all those who are engaged in the handling and production of fish and fishery products, or are concerned with their storage, distribution, export, import and sale in attaining safe and wholesome products which can be sold on national or international markets and meet the requirements of the CODEX Alimentarius Commission Standards.
This Code of Practice was approved by the Thirtieth Meeting of the Council for Trade and Economic Development (COTED) on 3-4 May 2010.
1This Code of Practice applies to the growing, harvesting, handling, production, processing, storage transportation and retail sale of fish, shellfish and aquatic invertebrates and products from marine and freshwater sources, which are intended for human consumption.
The following reference documents are indispensible for the application of this document. The latest edition of the referenced documents (including any amendments) applies.
CARICOM Regional Code of Practice, CRCP 5, General principles for food hygiene
CARICOM Regional Standard, CRS 5, Labelling standard for pre-packaged foods
CODEX Alimentarius Commission, Code of Practice for Fish and Fishery Products
CODEX Alimentarius Commission, General Standard for Food Additives (CODEX STAN 192-1995)
CODEX Alimentarius Commission, Guidelines for Sensory Evaluation of Fish and Shellfish in Laboratories (CAC/GL 31-1999)
CODEX Alimentarius Commission, Guidelines for the Establishment of a regulatory programme for control of veterinary drugs residues in foods (CAC/GL 16-1993)
CODEX Alimentarius Commission, Methods of Sampling and Analysis (CODEX STAN 234-1999)
CODEX Alimentarius Commission, Methods for Determination of Glaze
CODEX Alimentarius Commission, Recommended Code of Practice on Good Animal Feeding (CAC/RCP 54 – 2004)
CODEX Alimentarius Commission, Standard for Fats and Oils not covered by Individual Standards (CODEX STAN 19-1981)
CODEX Alimentarius Commission, Standard for Frozen Fish Fingers, Fish Portions and Fish Fillets – Breaded or in Batter (CODEX STAN 166-1989)
CODEX Alimentarius Commission, Standard for Named Vegetable Oils (CODEX STAN 210-1999)
CODEX Alimentarius Commission, Standard for Olive Oils and Olive Pomace Oils (CODEX STAN 33-1981)
CODEX Alimentarius Commission, Standard for Quick Frozen Blocks of Fish Fillet, Minced Fish Flesh and Mixtures of Fillets (CODEX STAN 165-1989)
CODEX Alimentarius Commission, Standard for Quick Frozen Fish Fillets (CODEX STAN 190-1995)
FAO listing of shrimps, FAO Fisheries Synopsis No. 125, Volume 1, Shrimps and Prawns of the World
Food and Agriculture Organization, 1995, Code of Conduct for Responsible Fisheries
OIE Codes of Practice, 2003, 6th Edition, International Aquatic Animal Health Code
Recommended International Code of Hygienic Practice for Low-Acid and Acidified Low- Acid Canned Food (CAC/RCP 23-1979)
2Recommended International Code of Hygienic Practice for the Transport of Food in Bulk and Semi-Packaged Food (CAC/RCP 47-2001)
Recommended International Code of Practice-General Principles of Food Hygiene, Section VIII-Transportation, CAC/RCP 1-1969
Recommended International Code of Practice for Control of the Use of Veterinary Drugs (CAC/RCP 38-1993)
For the purposes of this Code of Practice, the following terms and definitions shall apply.
poisonous substances naturally present in fish and fishery products or accumulated by the animals feeding on toxin producing algae, or in water containing toxins produced by such organisms
process of cooling fish and shellfish to a temperature between 0°C and 4 °C
water from any source where harmful microbiological contamination, substances and or toxic plankton are not present in such quantities as may affect the quality of fish, shellfish and their products
removal of soil, food residues, dirt, grease or other objectionable matter
biological or chemical agent, foreign matter, or other substance not intentionally added to food which may compromise food safety or suitability
introduction or occurrence of a contaminant in fish, shellfish and their products
action and activity that can be used to prevent or eliminate a food safety hazard or reduce it to an acceptable level
NOTE For the purposes of this Code of Practice, a control measure is also applied to a defect.
action to be taken when the results of monitoring at the CCP indicate a loss of control
NOTE For the purposes of this Code of Practice, this also applies to a Defect Action Point (DAP).
3step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level
criterion, which separates acceptability from unacceptability
NOTE For the purposes of this Code of Practice, this also applies to a DAP.
sequence of questions applied to each process step, with an identified hazard to determine which process steps are CCPs
NOTE For the purposes of this Code of Practice this also applies to a DAP.
deterioration of fish, shellfish and their products including texture breakdown and causing a persistent and distinct objectionable odour or flavour
condition found in a product which fails to meet essential quality, composition and or labelling provisions of the appropriate national, regional or international product standards
step at which control can be applied and a quality (non-safety) defect can be prevented, eliminated or reduced to acceptable level, or a fraud risk eliminated
reduction, by means of chemical agents and or physical methods, of the number of micro-organisms in the environment, to a level that does not compromise food safety or suitability
portion of fish remaining after heading and gutting
premises where fish and fishery products are prepared, processed, chilled, frozen, packaged or stored.
NOTE For the purposes of this Code of Practice, premises also include vessels.
cold-blooded (ectothermic) aquatic vertebrate excluding amphibians and aquatic reptiles
biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect
4process of collecting and evaluating information on hazards and conditions leading to their presence to decide which are significant for food safety
system which identifies, evaluates, and controls hazards which are significant for food safety
act of conducting a planned sequence of observations or measurements of control parameters to assess whether a CCP is under control
water, fit for human consumption and free from micro-organisms of public health significance and harmful toxic substances
programme that is required prior to the application of the HACCP system to ensure that a fish and shellfish processing facility is operating according to the CARICOM Regional Code of Practice, CRCP 4, General principles for food hygiene and appropriate national food safety legislation
fresh and frozen fish, shellfish and or their parts which may be utilised to produce fish and shellfish products intended for human consumption
clean water cooled by a suitable refrigeration system
period during which the product maintains its microbiological and chemical safety and sensory qualities at a specific storage temperature
NOTE It is based on identified hazards for the product, heat or other preservation treatments, packaging method and other hurdles or inhibiting factors.
species of aquatic molluscs and crustaceans that are commonly used for food
point, procedure, operation or stage in the food chain including raw materials, from primary production to final consumption
obtaining evidence that the elements of the HACCP plan are effective
5application of methods, procedures, tests and other evaluations, in addition to monitoring, to determine compliance with the HACCP plan
NOTE For the purposes of this Code of Practice, this also applies to a DAP.
fish as captured, un-gutted
NOTE Also known as round fish.
farming during part of or the whole life cycle of all aquatic animals, except mammalian species, aquatic reptiles and amphibians, intended for human consumption, but excluding bivalve molluscs
NOTE These aquatic animals are hereafter referred to as “fish”.
premises for the production of fish intended for human consumption, including the supporting inner infrastructure and surroundings under the control of the same management
substance either natural or synthetic which can affect the live fish, its pathogens, the water, equipment used for production or the land within the aquaculture establishment
obtaining specifically coloured feature of a targeted organism by incorporating into the fish food a natural or artificial substance or additive approved for this purpose by the national competent authority
EXAMPLE Coloured feature include flesh, shell and gonad
fish on or in which pathological changes or other abnormalities that affect safety and quality are apparent
raising fish under conditions of little or incomplete control over the growing process and production conditions
chemicals other than nutrients which are approved for fish
aquaculture production unit usually consisting of holding facilities, plant, service equipment and stock
| EXAMPLE 1 | Production units may be either land or water based 6 |
| EXAMPLE 2 | Holding facilities include tanks, ponds, raceways and cages |
| EXAMPLE 3 | Plant includes buildings, storage and processing areas |
fodder intended for fish in aquaculture establishments, in any form and of any composition
practices of the aquaculture sector that are necessary to produce quality and safe food products conforming to food laws and regulations
NOTE Also known as good fish farming practices.
operations involving removing the fish from the water
raising fish under controlled growing process and production conditions where their growth is completely dependent on externally supplied fish feed
official authority or authorities charged by the government with the control of food hygiene and sanitation in aquaculture
substance intended for preventing, destroying, attracting, repelling or controlling any pests during the production, storage, transport, distribution and processing of food, agricultural commodities, or animal feeds or which may be administered to animals for the control of ectoparasites
NOTE The term normally excludes fertilisers, plant and animal nutrients, food additives, and veterinary drugs.
specified substance in food, agricultural commodities, or animal feed resulting from the use of a pesticide
NOTE The term includes any derivatives of a pesticide, such as conversion products, metabolites, reaction products, and impurities considered to be of toxicological significance.
foreign substance including its metabolites, which remains in fish prior to harvesting as a result of either application or accidental exposure
raising fish under conditions of partial control over the growing process and production conditions where their growth is dependent upon endogenously supplied nutrient inputs and externally supplied fish feed
7amount of fish stocked per unit of area or volume
substance applied or administered to any food-producing animal, such as meat or milk-producing animals, poultry, fish or bees, whether used for therapeutic, prophylactic or diagnostic purposes or for modification of physiological functions or behaviour
period of time necessary between the last administration of a veterinary drug to fish, or exposure of these animals to a veterinary drug, and harvesting of them to ensure that the concentration of the veterinary drug in their edible flesh intended for human consumption, complies with the maximum permitted residue limits
approved by the competent authority
placing live bivalve molluscs in tanks, floats or natural sites to remove sand, mud or slime and improve product acceptability
reduction of microorganisms to a level acceptable for direct consumption by the process of holding live bivalve molluscs for a period of time under approved, controlled conditions in natural or artificial, treated or untreated sea water suitable for the process
approved establishment for the depuration of live bivalve molluscs
approved on-shore or off-shore installation or establishment for the reception, conditioning, washing, cleaning, grading and packaging of live bivalve molluscs fit for human consumption from which the bivalve molluscs are dispatched alive
brackish and marine areas approved for the production or harvesting of bivalve molluscs either by natural growth or by aquaculture destined for human consumption.
NOTE The growing areas may be approved as production or harvesting areas for bivalve molluscs for direct consumption, or they may be approved as production or harvesting areas for bivalve molluscs for either depuration or relaying.
process of subjecting bivalve molluscs in the shell to any form of heat treatment, such as steam, hot water, or dry heat for a short period of time, to facilitate rapid removal of meat from the shell for the purpose of shucking
8removal of bivalve molluscs from microbiologically contaminated growing area to an acceptable growing or holding area under the supervision of the competent authority and holding them there for the time necessary for the reduction of contamination to an acceptable level for human consumption
process of removing the edible portion of the mollusc from the shell by hand, mechanically or through heat shock with steam or hot water
passing fillets over a translucent table illuminated from below to detect parasites and other defects
loss of moisture from frozen products through evaporation
NOTE This may occur if the products are not properly glazed, packaged or stored. Deep dehydration adversely affects the appearance and surface texture of the product and is commonly known as “freezer burn”.
slice of fish of irregular size and shape removed from the carcass by cuts made parallel to the backbone
equipment designed for freezing fish and other food products, by quickly lowering the temperature so that, after thermal stabilisation, the temperature in the thermal centre of the product is the same as the storage temperature
process which is carried out in an appropriate equipment in such a way that the range of temperature of maximum crystallisation is passed quickly
facility that is capable of maintaining the temperature of fish at −18 °C
fish or fishery products which have received no preserving treatment other than chilling
fish which have been subjected to a freezing process sufficient to reduce the temperature of the whole product to a level low enough to preserve the inherent quality of the fish and which have been maintained at this low temperature during transportation, storage and distribution up to and including the time of final sale
NOTE For the purposes of this Code of Practice, the terms “frozen”, “deep frozen”, “quick frozen”, unless otherwise stated, are synonymous.
9applications of a protective layer of ice formed at the surface of a frozen product by spraying it with, or dipping it into, clean sea water, potable water, or potable water with approved additives
comminuted flesh produced by separation from skin and bones
packaging in which the atmosphere surrounding the fish is different from the normal composition of air
mechanical process for producing minced fish whereby the skin and bone are substantially removed from the flesh
mechanical device used for separation
section of fish, removed by cutting approximately at right angle to the backbone
removal of excessive wash water from the minced fish flesh
fish protein product for further processing, which has been processed by heading, gutting, cleaning fresh fish and mechanically separating the edible muscle from the skin and bone which is then minced, washed, refined, de-watered mixed with cryoprotective food ingredients and frozen
ability of surimi to form an elastic gel when fish meat is comminuted with the addition of salt and then heated
NOTE This elasticity is a function possessed by myosin as the primary component of myofibrillar protein.
generic term of skeletal muscle proteins such as myosin and actin
process of removing from washed meat, by use of a strainer, small bones, sinews, scales and bloody flesh of such sizes as may not be mixed in a final product, thereby concentrating myofibrillar protein
10variety of products produced from surimi with addition of ingredients and flavour such as “surimi gel” and shellfish analogues
fish meat that is washed and then drained of water
process of removing blood and water soluble components from minced fish with cold water by the use of a rotary filter, thus increasing the level of myofibrillar proteins thereof
water-soluble protein, organic substance and inorganic salts contained in fish meat
liquid preparation from ground cereals, spices, salt, sugar and other ingredients and or additives for coating
NOTE Typical batter types include non-leavened batter and leavened batter.
dry breadcrumbs or other dry preparations mainly from cereals with colourants and other ingredients used for the final coating of fishery products
NOTE Typical breading types include free-flowing breading, coarse breading, flour-type breading.
covering the surface of a fishery product with batter and or breading
frying of breaded and battered fishery products in an oil bath in a way so that the core remains frozen
cutting of regular shapes of fish blocks into pieces suitable for later coating
cylindrical container made from wood, plastic or other suitable food contact material with a lid for water-tight closure
parietal peritoneum; the pigmented lining of the abdominal cavity
11solution of salt in water
process of injecting brine directly into the flesh of the fish
process of placing fish in brine for a period of time, sufficient for the fish tissue to absorb a specific quantity of salt
process of mixing fish with suitable food grade salt and stacking the fish in such a manner that the resulting brine drains away
discolouration and development of the mould Sporendonema epizoum, which affect the fish surface and gives it a peppered appearance
NOTE The fish flesh is unaffected.
fish in which the main reserves of fat are in the body tissue and the fat content is more than 2 %
process of removing the gills, long gut and stomach from fatty fish, such as herring, by inserting a knife or using hands at the gills; the milt or roe and some of the pyloric caeca are left in the fish
fish in which the main reserves of fat are in the liver and less than 2 % fat in the body tissue
NOTE Also known as white fish.
process from the initial salting until the fish is salt-matured
removing the head and gut from fatty fish, such as herring, in one operation by partially severing the head and pulling the head away together with attached gut; the roe or milt is left in
brine which may contain vinegar and spices
process whereby primary fatty fish is mixed with suitable salt which may contain vinegar and spices and stored in watertight containers under the resultant pickle which forms by solution of salt in the water extracted from the fish tissue
12NOTE Pickle may be added to the container. Pickled products will always remain in a brine solution.
discolouration caused by red halophilic bacteria which damages the flesh of the fish
crystalline product consisting predominantly of sodium chloride
NOTE It is obtained from the sea, from underground rock salt deposits or from vacuum processed and refined brine.
salted fish that has an appearance, consistency and flavour characteristic of the final product
fish or fillets which have been treated by either brining, brine injection, dry-salting, pickling or wet-salting, or a combination of these
water phase of the fish muscle which is saturated with salt (26.4 g salt/100 g water phase).
fish that have been cut open from throat or nape to the tail, with gills, guts, roe or milt removed
NOTE Head and whole or part of backbone may be left in or removed.
laying fish in piles with salt spread evenly on the surface
process whereby primary lean fish is mixed with suitable food grade salt and stored in watertight containers under the resultant brine which forms by solution of salt in the water extracted from the fish tissue
NOTE Brine may be added to the container. The fish can be removed from the container and stacked so that the brine drains away.
removing the head from the entire shrimp or prawn
shrimp which has been peeled; the back of the peeled segments of the shrimp have been opened out and the gut (vein) removed
freshly caught shrimp which has been chilled or has not been otherwise preserved
13NOTE This does not include freshly cooked shrimp.
shrimp with head and shell removed
raw shrimp with head removed and the shell left intact
species covered by the most recent edition of the FAO listing of shrimps, FAO Fisheries Synopsis No. 125, Volume 1, Shrimps and Prawns of the World.
NOTE Also called prawn.
process of cutting cephalopods along the mantle to produce a single fillet
commercially sterile food in hermetically sealed containers
condition achieved by application of heat, sufficient, alone or in combination with other appropriate treatments, to render the food free from micro-organisms capable of growing in the food at normal non-refrigerated conditions at which the food is likely to be held during distribution and storage
containers which are sealed to protect the content against the entry of microorganisms during and after heat treatment
pressure vessel designed for thermal processing of food packed in hermetically sealed containers
thermal process chosen by the processor for a given product and container size to achieve at least commercial sterility
NOTE Also known as sterilisation schedule.
temperature maintained throughout the thermal process as specified in the scheduled process
time between the moment sterilisation temperature is achieved and the moment cooling started
14heat treatment to achieve commercial sterility and is quantified in terms of time and temperature
thorough removal of the air from steam retorts by steam prior to a scheduled process
operation that stores, prepares, packages, serves, or otherwise provides fish, shellfish and their products directly to the consumer
NOTE This may be free standing seafood markets, seafood sections in grocery or department stores, packaged chilled or frozen and or full service.
placed in a container and displayed chilled or frozen for direct consumer pick-up
display of chilled fish, shellfish and their products to be weighed and wrapped by establishment personnel at the request of the consumer
Prior to the application of HACCP to any segment of the product processing chain, that segment should be supported by pre-requisite programmes based on good hygienic practices or as required by the competent authority.
NOTE 1 The establishment of pre-requisite programmes will allow the HACCP team to focus on the HACCP application to food safety hazards which are directly applicable to the product and the process selected, without undue consideration and repetition of hazards from the surrounding environment. The pre-requisite programmes would be specific within an individual establishment or for an individual vessel and will require monitoring and evaluation to ensure their continued effectiveness.
NOTE 2 HACCP principles can also be applied to defect action points.
The design and construction of a fishing vessel, and vessels used to harvest farmed fish and shellfish should take into consideration the following:
EXAMPLE CO2, O2, temperature and nitrogenous wastes
The facility should be designed to facilitate rapid processing and subsequent storage taking into consideration the following:
The condition of the equipment and utensils should be such that it minimises the build-up of residues and prevents them from becoming a source of contamination. The design and construction of equipment and utensils should take into consideration the following:
Schedules should be implemented to:
The hygiene control programme should take into consideration the following:
NOTE Pest control programmes could include preventing access, eliminating harbourage and infestations, and establishing monitoring detection and eradication systems.
Personal hygiene and facilities should ensure that an appropriate degree be maintained to avoid contamination.
Facilities and equipment should include:
Personal hygiene should be as follows:
Vehicles should be designed and constructed:
Product traceability and recall procedures should take into consideration the following:
21All personnel should be aware of their role and responsibility in protecting fish or shellfish from contamination and deterioration.
Handlers should have the necessary knowledge and skill to enable them to handle fish or shellfish hygienically.
Personnel who handle strong cleaning chemicals or other potentially hazardous chemicals should be instructed in safe handling techniques.
Each fish and shellfish facility should ensure that managers arrange for adequate and periodic training of relevant employees in the principles and application of HACCP.
NOTE Training of personnel in the use of HACCP is fundamental to the successful implementation and delivery of the programme in fish or shellfish processing establishments. The practical application of such systems will be enhanced when the individual responsible for HACCP has successfully completed a course.
Fish, shellfish and other aquatic invertebrates should not be accepted if they are known to contain parasites, undesirable microorganisms, pesticides, veterinary drugs or toxic, decomposed or extraneous substances known to be harmful to human health, unless they can be reduced to an acceptable level by normal sorting and or processing.
When fish and shellfish determined as unfit for human consumption are found, they should be removed and stored separately from the catch and either reworked and or disposed of in a proper manner.
All fish and shellfish deemed fit for human consumption should be handled properly with particular attention being paid to time and temperature control.
Fresh fish, fillets, shellfish and their products which are to be chilled should be held at a temperature as close as possible to 0 °C.
22NOTE Temperature is the single most important factor affecting the rate of fish and shellfish deterioration and multiplication of micro-organisms. For species prone to scombrotoxin production, time and temperature control may be the most effective method in controlling food safety.
To minimise the deterioration, it is important that:
Where temperature control is concerned:
To minimise handling damage:
EXAMPLE CO2, O2, temperature and nitrogenous wastes
NOTE Poor handling practices can lead to damage of fresh fish, shellfish and other aquatic invertebrates which can accelerate the rate of decomposition and increase unnecessary post-harvest losses.
An effective HACCP system should reduce the reliance on traditional end-product testing. The HACCP plan, which should be incorporated into the food management plan should be well documented and be as simple as possible. This section will demonstrate one format, which may be considered in the development of the HACCP plan. The establishment should follow the guidelines outlined in Annex C on how to use the principles of HACCP in the production of various fishery products.
Aquaculture establishments should comply with the recommendations of the Food and Agriculture Organization, Code of Conduct for Responsible Fisheries, 1995 in order to minimize any adverse impacts on human health and environment.
Fish farms should operate effective fish health and welfare management. Fry and fingerlings should be disease free and should comply with the OIE Codes of Practice (International Aquatic Animal Health Code, 6th Edition, 2003).
Growing fish should be monitored for disease.
When using chemicals at fish farms, special care should be exercised so that these substances are not released into the surrounding environment.
It should be recognised that in preparing a HACCP and or DAP plan it is essential to consult Annex C which provides guidance for the application of the principles of HACCP and DAP analysis.
NOTE See Annex D for an example of a flow diagram (figure D.2) for some of the common steps in aquaculture production.
The general principles in 4 apply to aquaculture production, in addition to the following:
The water should be suitable for the rearing of fish which are safe for human consumption.
The water quality should be monitored regularly such that the health of the fish and sanitary condition of the water are continuously maintained to ensure aquaculture products are safe for human consumption.
Fish farms should be sited where there is no risk of contamination of the water in which fish are reared.
Fish farms should be appropriately designed and constructed to ensure control of hazards and prevention of water contamination.
The source of post-larvae, fries and fingerlings should be such to avoid the carryover of potential hazards into the growing stocks.
Potential hazards that are specific to aquaculture products include: residues of veterinary drugs in excess of recommended guidelines and other chemicals used in aquaculture production, contamination of faecal origin where the facilities are close to human habitation or animal husbandry (see Annex C.4.1).
During transport of live fish stress should be reduced and care should be taken to minimise physical damage to fish (see Annex C.4.1).
25Feeds used in aquaculture production should be in accordance with the relevant national requirements and or the CODEX Alimentarius Commission Recommended Code of Practice on Good Animal Feeding (CAC/RCP 54 – 2004).
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Feed supply should be in accordance with the following:
Potential hazards include, but are not limited to residues of veterinary drugs.
Potential defects are unlikely.
The use of veterinary drugs should be in accordance with the following:
Potential hazards include, but are not limited to, microbiological and chemical contamination.
Potential defects include, but are not limited to:
Growing of fish should be in accordance with the following:
Potential hazards are unlikely.
28Potential defects include, but are not limited to:
Harvesting of fish should be carried out in accordance with the following:
Potential hazards include, but are not limited to, microbiological and chemical contamination.
Potential defects include, but are not limited to:
Holding and transportation of fish should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
EXAMPLE Oil, cleaning and disinfecting agents
Potential defects include, but are not limited to:
Storage and transport of live fish should be in accordance with the following:
Potential hazards include, but are not limited to:
EXAMPLE Oil, cleaning and disinfecting agents
Potential defects include, but are not limited to:
Storage and transportation of live fish at ambient temperature should be in accordance with the following:
NOTE It may be necessary to insulate the holding tanks and install a temperature control system.
NOTE An alternative method is reduction of temperature.
Potential hazards include, but are not limited to:
EXAMPLE Oil, cleaning and disinfecting agents
Potential defects include, but are not limited to:
Storage and transportation of live fish at low temperature should be in accordance with the following:
NOTE Conditioning is a biological operation to reduce the metabolic rate of fish thereby minimising the stress caused by storage and transportation.
NOTE There is a range of temperature in which fish do not exhibit or have reduced physical activity. The limit is attained at the temperature at which the metabolic rate of the fish is minimised without causing adverse effects to them.
Annex C outlines the application of HACCP principles and Annex E provides additional information for pre-requisite programmes for live and raw bivalve molluscs.
Potential hazards include, but are not limited to:
NOTE There are five different types of important hazards coming from the bivalve molluscs growing environment:
EXAMPLE Salmonella spp.
EXAMPLE Norovirus, viruses causing hepatitis
EXAMPLE Vibrio spp
EXAMPLE okadaic acid group (DSP), saxitoxin group (PSP), brevetoxin group (NSP), domoic acid group (ASP), azaspiracid group (AZP)
EXAMPLE heavy metals such lead, cadmium and mercury
Potential defects are unlikely.
33Surveys of the growing area, shoreline and land catchment should be conducted to determine sources of both domestic and industrial pollution which may affect the quality of the growing area water and bivalve molluscs. Resurveys should be conducted at an acceptable frequency and known pollution sources should be re-evaluated on a regular basis to determine any changes to their impact on the growing area.
NOTE 1 Sources may include municipal sewage outputs, industrial outputs, mine wastes, geophysical contaminants, domestic animal holding pens, nuclear power plants, refineries or other sources.
NOTE 2 The need to reschedule hygiene surveys will be determined by population shifts and changes in agricultural and industrial activities in the coastal area.
When pollution sources have been identified and evaluated, sampling stations for water and or bivalve molluscs and or sediments should be established and studies conducted to determine the effects of the pollutants on water and bivalve molluscs quality. The data should be evaluated by the national competent authority and growing areas should be classified according to national requirements and criteria.
When interpreting growing area data, the national competent authority should take into account variations which may affect the level of pollution during the most unfavourable hydrographical and climatic conditions as influenced by rainfall, tides, winds, methods of sewage treatment, population variations and other local factors. The authority should also consider that bivalve molluscs have the ability to accumulate toxic chemicals in their tissue in concentrations greater than the levels found in the surrounding water.
NOTE FAO, WHO, or other international or national food standards may be used as a guide to acceptable levels.
The national competent authority should immediately announce decisions concerning the classification of growing areas to the affected producers, depuration and distribution centres.
When sampling shellfish meats for classification purposes, if the limits of any biological or chemical hazard stipulated by the national competent authority are exceeded, appropriate measures should be taken under the responsibility of the national competent authority.
Classified growing areas should be clearly defined by the national competent authority as either:
Growing areas should be routinely monitored for changes in water quality and or bivalve molluscs quality, and sub-standard areas patrolled to prevent harvesting for purposes other than that established by the national competent authority.
NOTE For early warning purposes, where appropriate, it is recommended to have a programme present to monitor growing areas for the species of plankton that can produce toxins and to recognize other environmental signals that a toxic event may be developing.
Harmful chemical substances within bivalve molluscs should not be present in amounts so that the calculated dietary intake exceeds the permissible daily intake.
34When routine monitoring programmes or resurveys show that the growing area no longer meets the classification criteria, the area should be reclassified or closed for harvesting immediately by the national competent authority.
In determining the public health suitability of bivalve molluscs classified growing areas, the national competent authority should consider the following actions:
Under the responsibility of the national competent authority, the growing areas providing bivalve molluscs for direct human consumption should meet the following requirements at time of harvest:
NOTE This can be determined by examination of mollusc’s flesh or through adequate monitoring of the water, as appropriate.
All growing water and or molluscan flesh should be monitored for the presence of E. coli, faecal coliforms or total coliforms at an appropriate frequency based on the probability and degree of faecal contamination.
Tests for suitable indicator bacteria such as faecal coliforms or E. coli, total coliforms should be used to determine the degree of faecal contamination. The effectiveness of indicator bacteria used should be kept under constant review for their reliability as measures for the degree of faecal contamination. These indicators do not correlate well with the presence of viruses, other controls such as shoreline surveys should always be employed If faecal contamination exceeds a certain threshold-level relaying or depuration for a time approved by the national competent authority may be allowed.
NOTE Other methods such as bacteriophage and viral detection could also be used as indicators when validated analytical methods become available in the future.
The species, and typically the actual strain of the pathogen should be known to ensure that monitoring addresses the source of the pathogen. Predetermined acceptance or rejection levels for the pathogen should be established in order to use such monitoring results for decision making. Other conditions including the sanitary survey requirements should be satisfied for the reopening of the harvest area.
35NOTE Shellfish sanitation programs rely upon the use of indicator organisms for the presence of contamination rather than upon attempts to monitor for specific pathogens. However, where there has been a shellfish borne outbreak caused by an identified pathogen such as Salmonella and others (Vibrio and viruses), monitoring the bivalve molluscs may be appropriate as part of the process of closure or reopening the affected harvest area.
Growing areas should also be monitored for environmental signals of toxicity such as, dead or dying birds, mammals, or fish. The risk of toxic algae blooms should be recognised when drawing up monitoring schedules.
NOTE 1 Toxic algae blooms show seasonal variability. Areas may also be affected by toxic algae previously unknown in the surrounding sea or coastal waters.
NOTE 2 Phytoplankton monitoring is a valuable complementary tool that can be used, in combination with the required monitoring of marine biotoxins in shellfish tissue, to optimize program management and resources.
The implication that the absence of toxicity in indicator shellfish species signifies the absence of toxicity of other species in the growing area should be verified for each species and for each group of toxins before defining a particular species as an indicator for that growing area.
The national competent authority should immediately close and effectively patrol affected areas when acceptable levels of toxins are exceeded in edible portions of bivalve molluscs meats. These areas should not be re-opened before a toxicological investigation has revealed that the bivalve molluscs meat is free from hazardous amounts of biotoxins.
The national competent authority should immediately announce these decisions to the affected producers, depuration and distribution centres.
In establishing a sampling programme, consideration should be given to ensure an adequate location and number of sampling sites. Sampling frequency should be sufficient to address spatial-temporal changes in micro-algae, toxins in shellfish and to cover the risks of rapid rises in shellfish toxicity.
NOTE Testing for a particular biotoxin may not be appropriate when it has been demonstrated that this biotoxin has not been associated with bivalve molluscs in the growing and harvesting areas.
The selection of sampling stations for both benthic and suspended culture should be based on sites which have historically presented toxicity in the early stages of a toxic event In order to protect public health, the selection of sampling stations should give appropriate coverage of the extent of a toxic event or the likely “worst case scenario” in a growing area. This should be based on the following factors:
EXAMPLE Toxic micro-algae from cyst beds
When a toxic event is in progress or developing, targeted, depth-specific sampling should be considered.
NOTE Routine sampling for micro-algae generally means taking an integrated sample from the water column.
Sampling for shellfish grown in suspension should at least involve an integrated sample composed of shellfish taken from the top, middle and bottom of the lines.
Minimum weekly sampling frequencies should be adopted by most monitoring programmes in areas where toxicity is prevalent and where harvesting is taking place or about to take place. Decisions on the frequency of sampling should be based on risk evaluation.
NOTE Inputs into the decision may include factors such as seasonality (toxicity and or harvesting), accessibility, historical baseline information, including toxin and micro-algal data, and the effects of environmental factors such as wind, tide and currents.
Sampling frequency and the factors that may lead to changes should be described in a “Marine Biotoxin Action Plan” for the growing area.
The number of shellfish sampled should be sufficient to address the variability of toxicity among individual shellfish. The number of shellfish in the sample, rather than the mass of the shellfish flesh should be the determining factor for the sample size. Additionally, the size of the sample should be sufficient to allow the test or tests for which the sample is being taken to be carried out, and the shellfish sampled should be of the size marketed.
NOTE There is no internationally agreed sample size for different shellfish species.
Methods suitable for the determination of marine biotoxins should be approved by the national competent authority.
Growing areas should be monitored for chemical contaminants on a sufficiently frequent basis to provide confidence that any identified sources of chemical contamination are not contaminating the shellfish. Shellfish growing areas where there are no known point sources of likely chemical contamination should only require occasional checks every few years. Where there are known point sources of specific contamination, shellfish should be checked more frequently on a routine basis. There should also be the capacity to sample shellfish reactively if a defined event occurs such as spillage of anti-fouling paint.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to, physical damage.
The harvesting and transportation of live bivalve molluscs should be carried out in accordance with the following:
NOTE The water could be re-circulated if it meets the definition for clean water.
The requirements for classification and monitoring of growing areas should also apply to relaying areas.
38Bivalve molluscs harvested for relaying should only be harvested from areas that are so designated or classified by the national competent authority.
NOTE Relaying is intended to reduce the level of biological contaminants that may be present in bivalve molluscs which have been harvested from contaminated areas to such levels that the bivalve molluscs will be acceptable for human consumption without further processing.
Potential hazards include, but are not limited to:
Potential defects are unlikely.
Relaying should be carried out in accordance with the following:
Bivalve molluscs harvested for depuration should only be harvested from areas that are so designated or classified by the national competent authority.
NOTE Depuration is intended to reduce the number of pathogenic micro-organisms that may be present in bivalve molluscs which have been harvested from moderately polluted areas to such levels that the bivalve molluscs will be acceptable for human consumption without further processing. Depuration alone is not suitable for cleansing bivalve molluscs from more heavily contaminated areas or areas subject to contamination by hydro-carbons, heavy metals, pesticides, viruses, vibrios or biotoxins.
39For natural functioning and for depuration to occur, molluscs should not be over-stressed or damaged during harvesting or handling and should not be in a seasonally weak or spawning condition.
Depuration centres should maintain the same hygiene standards as outlined in 4.3, 4.4, 4.5 and 4.6.
Potential hazards include but are not limited to, microbiological contamination.
Potential defects include, but are not limited to, physical damage.
Depuration should be carried out in accordance with the following:
NOTE Viruses and Vibrio spp. are more persistent during depuration than the indicator bacteria mostly used for microbiological monitoring and that the reducing of the number of indicator bacteria does not always reflect the real situation with respect to contamination by viruses and Vibrio spp.
Distribution centres that prepare live bivalve molluscs suitable for direct consumption and establishments that prepare live and raw bivalve molluscs suitable for direct consumption should maintain the same hygiene standards as 4.3, 4.4, 4.5 and 4.6.
Potential hazards include, but are not limited to, microbiological, chemical and physical contamination.
Potential defects include, but are not limited to:
Reception of bivalve molluscs should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Conditional storage of bivalve molluscs should be carried out in accordance with the following:
NOTE Optimum salinity will vary with bivalve molluscs species and with the harvesting area. Water condition has to be satisfactory adequate for the process.
Potential hazards include, but are not limited to microbiological contamination, chemical and physical contamination.
Potential defects include, but are not limited to mechanical damage.
Washing, declumping, debyssing and grading should be carried out in accordance with the following:
All steps in the process of packaging should be performed without delay and under conditions that will prevent the possibility of contamination, deterioration and the growth of pathogenic and spoilage micro-organisms.
The packaging material should be appropriate for the packaging and storage of the product and should not transmit to the product harmful or other objectionable substances or odours and tastes. The packaging material should be sound and should provide appropriate protection from damage and contamination.
Potential hazards include, but are not limited to microbiological, physical and chemical contamination.
43Potential defects include, but are not limited to:
Packing and labelling of live bivalve molluscs should be carried out in accordance with the following:
NOTE The packaging material may be used to bear an indication as to how the bivalve molluscs should be kept at the time they were bought at the retailer. It is recommended to include the date of packaging.
Potential hazards include, but are not limited to, microbiological and physical contamination.
Potential defects include, but are not limited to:
Packing and labelling of raw bivalve molluscs should be carried out in accordance with the following:
NOTE The packaging material or label may be used as a means to convey appropriate storage instructions to the consumer after retail purchase. It is recommended to include the date of packaging.
44NOTE Slow freezing will damage meat.
Potential hazards include, but are not limited to microbiological contamination, chemical and physical contamination.
Potential defects include, but are not limited to physical damage.
The storage of live bivalve molluscs should be carried out in accordance with the following:
Potential hazards include, but are not limited to, microbiological contamination, chemical and physical contamination.
Potential defects include, but are not limited to physical damage.
The storage of raw bivalve molluscs should be carried out in accordance with the following:
Potential hazards include, but are not limited to, microbiological contamination.
Potential defects include, but are not limited to, physical damage.
The distribution and transport of live bivalve molluscs should be carried out in accordance with the following:
NOTE See also 4.7 and 16.
Potential hazards include, but are not limited to, microbiological contamination.
Potential defects are unlikely.
The distribution and transportation of raw bivalve molluscs should be carried out in accordance with the following:
Live and raw bivalve molluscs should meet all microbiological criteria associated with traditional harvest water controls designed to prevent faecal contamination and resulting introduction of enteric pathogens as well as toxins and other contaminants.
NOTE Processing to reduce or limit target microorganisms is intended to retain the sensory qualities of a live bivalve mollusc. However, these growing area controls are not designed for control of pathogens that are independent from faecal contamination.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Processing to reduce or limit target organisms should be carried out in accordance with the following:
Dirt, mud and detritus should be removed before further processing through washing or other means.
Potential hazards include, but are not limited to, microbiological contamination and physical contamination.
Potential defects include, but are not limited to:
Shucking should be carried out in the accordance with the following:
Potential hazards include, but are not limited to physical contamination.
Potential defects are unlikely.
Heat shocking and packing of bivalve mollusc should be carried out in the accordance with the following:
The transport of live bivalve molluscs from a growing area to a distribution centre, depuration centre, relaying area or establishment should be accompanied by documentation for the identification of batches of live bivalve molluscs.
Storage and transport temperatures should be indicated.
Permanent, legible and dated records of relaying and depuration, pertaining to each lot, should be retained for a minimum period of one year.
Depuration centres or tanks and distribution centres and establishments should only accept lots of live bivalve molluscs with documentation issued by or accepted by the national competent authority. Where appropriate, this documentation should contain the following information:
Complete records of harvest area and date of harvest and length of time of relaying or depuration of each lot should be maintained by the distribution centre or establishment for a period designated by the national competent authority.
Each product should have an easy identifiable lot number. This lot number must include an identification code, the number of the establishment that distributes the product, the country of origin and day and month of packing, in order to facilitate the traceability or product tracing of the product. A record keeping system should be based on these lot numbers so that individual lots of bivalve molluscs can be traced from the growing area to the end user.
NOTE See also 4.8.
Annex C provides the application of HACCP principles and additional information for prerequisite programmes for processing of fresh, frozen and minced fish.
Processing of fresh fish in a MAP product, or minced or frozen fish, is used as the basis for all the other fish processing operations (see 11 to 15).
NOTE See Annex D for the example of the flow diagram which will provide guidance to some of the common steps involved in a fish fillet preparation line.
The hygienic conditions and technical manner in which fish are prepared are not greatly influenced by its intended purpose of direct distribution or further processing. Variations will exist in the form in which the fresh fish flesh is to be utilised. The forms may include, but are not limited to, dressed, fillets or steaks.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Raw, fresh or frozen fish reception should be carried out in the accordance with the following:
EXAMPLE TVBN, histamine, heavy metals, pesticide residues, nitrates
The best method of assessing the freshness or spoilage of fish is by sensory evaluation techniques as established in CAC/GL 31-1999. It is recommended that appropriate sensory evaluation criteria be used to evaluate the acceptability of fish, and to eliminate fish showing loss of essential quality provisions of the appropriate CODEX Alimentarius Commission standards. Fresh white fish species are considered unacceptable when showing the characteristics stated in Table 1.
| Sensory evaluation criteria | Characteristics |
|---|---|
| Skin / Slime | Dull, gritty colours with yellow brown dotting slime |
| Eyes | Concave, opaque, sunken discoloured |
| Gills | Grey – brown or bleached, slime opaque yellow, thick or clotting |
| Odour | Flesh odour amines, ammonia, milky lactic, sulphide, faecal, putrid, rancid |
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Chilled storage should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Frozen storage should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to, decomposition.
Control thawing should be carried out in the accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Washing and gutting should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
EXAMPLE skin and scales
Filleting, skinning, trimming and candling should be carried out in accordance with the following:
Potential hazards are unlikely.
Potential defects include, but are not limited to incorrect net weight.
Weigh scales should be periodically calibrated with a standardised mass to ensure accuracy.
Potential hazards include, but are not limited to:
EXAMPLE metal
Potential defects include, but are not limited to subsequent decomposition.
The extent to which the shelf-life of the product can be extended by vacuum or MAP will depend on the species, fat content, initial bacterial load, gas mixture, type of packaging material and the temperature of storage.
Modified atmosphere packaging should be strictly controlled by:
Packaging material should be inspected prior to use to ensure that it is not damaged or contaminated.
Packaging integrity of the finished product should be inspected at regular intervals by appropriately trained personnel to verify the effectiveness of the seal and the proper operation of the packaging machine.
Following sealing, MAP or vacuumed products should be transferred carefully and without undue delay to chilled storage.
Adequate vacuum should be attained, and the package seals should be intact.
Potential hazards are unlikely.
Potential defects include, but are not limited to incorrect labelling.
Labelling should be carried out in accordance with the following:
57Potential hazards include, but are not limited to, metal contamination.
Potential defects are unlikely.
Metal detection should be carried out in accordance with the following:
Potential hazards include, but are not limited to, viable parasites.
Potential defects include, but are not limited to:
The freezing process should be carried out in accordance with the following:
Potential hazards include, but are not limited to, microbiological pathogens.
Potential defects include, but are not limited to:
Glazing should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
EXAMPLE Metal, bones and rubber from separator belt
Potential defects include, but are not limited to:
EXAMPLE objectionable matter
Candling is recommended for fish suspected of high infestation with parasites prior to the mincing of fish using mechanical separation. The process of mincing of fish using mechanical separation should be carried out in the accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Washing of minced fish should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
The blending and application of additives and ingredients to minced fish should be carried out in accordance with the following:
Potential hazards include, but are not limited to, microbiological pathogens.
Potential defects include, but are not limited to:
Wrapping and packing should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to incorrect description.
Reception of packaging, labels and ingredients should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to, loss of quality characteristics of packaging materials or ingredients.
Storage of packaging, labels and ingredients should be carried out in accordance with the following:
Annex C provides the application of HACCP principles and additional information for prerequisite programmes for processing of frozen surimi.
Annex D provides an example of a flow chart for a frozen surimi production process.
63Many of the potential food safety hazards should be controlled during processing. Pathogenic bacteria such as Listeria monocytogenes and toxin formers such as Clostridium botulinum should be controlled during the cooking or pasteurising steps of final processing. Staphylococcus aureus contamination that produces heat-stable enterotoxins should be adequately controlled by the pre-requisite programme.
NOTE Clostridium botulinum becomes a hazard as a result of modified atmosphere packaging of the end product.
If scombrotoxin-forming fish that may accumulate ciguatera toxin are utilised for surimi, appropriate controls for this hazard should be developed.
Appropriate controls should be instituted to ensure that metal fragments such as bearings, bolts, washers, and nuts are excluded or eliminated in the end product.
Colour, moisture content, pH or gel strength are quality attributes of frozen surimi that are important for the successful manufacture of surimi-based products.
Species used that are known to contain Myxosporidia, a protease inhibitor, may be needed as an additive to attain the necessary gel strength capabilities.
Decomposed fish should not be used as raw material for frozen surimi production.
The washing and de-watering cycle should be sufficient to prevent water-soluble proteins remaining in the product as this may negatively affect the gel forming ability and the long term frozen storage shelf life.
Objectionable matter such as small bones, scales and black belly lining should be minimised as it negatively affects the usability of frozen surimi for processing into end products.
The use of additives to surimi should be in accordance with appropriate regulations and manufacturer’s recommendations.
Cold water marine fish should not be subjected to temperatures above 10 °C during processing to prevent protein denaturation.
See 9.2, steps 1 through 8 for information regarding preparation of fish for processing. For frozen surimi processing, consideration should be given to the steps outlined in the following sub-clauses.
Potential hazards are unlikely when using marine ground fish as the raw material.
64Potential defects include, but are not limited to:
Raw, fresh and frozen fish reception should be carried out in accordance with the following:
EXAMPLE An aggregate flesh for Alaska Pollock (Theragra chalcogramma) should have pH of 7.0 ± 0.5
Potential hazards are unlikely.
Potential defects include, but are not limited to protein denaturation.
Chilled storage should be carried out in accordance with the following:
65Potential hazards are unlikely.
Potential defects include, but are not limited to:
The epidermis, scales and loose pigment should be removed before heading and gutting. This will lessen the level of impurities and extraneous material that can negatively affect the gel strength capability and colour of the end product.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Headed and gutted fish should be re-washed so as to lessen the level of impurities and extraneous material that can negatively affect the gel strength capability and colour of the end product.
Potential hazards include, but are not limited to metal fragments.
66Potential defects include, but are not limited to impurities.
Fish flesh separation should be carried out in accordance with the following:
Potential hazards include, but are not limited to pathogenic microbial growth.
Potential defects include, but are not limited to:
Washing and de-watering should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
The refining process should be carried out in accordance with the following:
Potential hazards include, but are not limited to, pathogenic microbial growth.
68Potential defects include, but are not limited to:
The process of final de-watering should be carried out in accordance with the following:
NOTE Some warm water species may be processed at temperatures up to 15 °C.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
The process of mixing and addition of adjuvant ingredients should be carried out in accordance with the following:
NOTE Some warm water species may be processed at temperatures up to 15 °C.
Potential hazards include, but are not limited to, pathogenic microbial growth.
Potential defects include, but are not limited to:
Packaging and weighing should be carried out in accordance with the following:
NOTE See also 9.3.1 and 9.5.4.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
The freezing operation should be carried out in accordance with the following:
NOTE See 9.4.1 for general considerations for freezing fish and fishery products.
Potential hazards are unlikely.
Potential defects include, but are not limited to, damage to plastic bag and product.
Care should be taken to avoid damage to plastic bag and the product in order to avoid deep dehydration during long-term cold storage.
Potential hazards include, but are not limited to metal fragments.
Potential defects are unlikely.
71Metal detection equipment that is capable of sensing product that has become contaminated with metal fragments should be installed at the most appropriate place in the process to eliminate this hazard.
NOTE See 9.3.4 for general information.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Boxing and labelling should be carried out in accordance with the following:
NOTE See 9.3.3 and 9.5.4.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Frozen storage should be carried out in accordance with the following:
See 9.6.1 for the packaging and ingredient requirements for raw material reception.
See 9.6.2 for the packaging and ingredient requirements for raw material storage.
Annex C provides the application of HACCP principles and additional information for prerequisite programmes.
Annex D provides an example of a flow chart for the processing of coated fish products.
Annex C.3.4 provides additional identification of hazards and defects specific to quick-frozen coated fish and shellfish., aside from those described in this section.
Potential hazards include, but are not limited to:
NOTE See also C.3.4.2
Potential defects are outlined in the essential quality, labelling and composition requirements described in the relevant CODEX Alimentarius Commission Standard CODEX STAN 166-1989.
General addition to pre-requisite programme should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Reception of fish should be carried out in accordance with the following:
Potential hazards include, but are not limited to chemical, biochemical and microbiological contamination.
Potential defects include, but are not limited to:
Reception of other ingredients should be carried out in accordance with the following:
Potential hazards include, but are not limited to, foreign matter.
Potential defects include, but are not limited to, tainting of products.
Packaging material should be in accordance with the following:
75For details on frozen storage of fish, refer to 9.2.5.
For details on chilled storage of fish, refer to 9.2.4.
Potential hazards include, but are not limited to biological, physical and chemical contamination.
Potential defects include, but are not limited to:
Storage of raw material, other ingredients and packaging material should be carried out in accordance with the following:
Potential hazards are unlikely.
Potential defects include, but are not limited to incorrect dimension due to sawing of over-softened fish flesh for fish sticks.
Frozen fish block or fillet tempering should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Unwrapping and unpacking should be carried out in accordance with the following:
Potential hazards include, but are not limited to foreign material.
EXAMPLE metal or plastic parts of saws
Potential defects include, but are not limited to irregularly shaped pieces or portions.
Production of fish core should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to incorrect addition of additives.
The temperature of the product in the mixing process should be adequately controlled to avoid the growth of pathogenic bacteria.
NOTE See also 9.5.3.
Potential defects include, but are not limited to:
78Potential defects include, but are not limited to:
Forming should be carried out in accordance with the following:
Potential hazards are unlikely.
Potential defects include, but are not limited to adhering pieces or portions.
Separation of pieces should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
79Potential defects include, but are not limited to insufficient or excessive cover of coating.
Wet coating should be carried out in accordance with the following:
EXAMPLE temperature control, dumping liquid contents, regular or scheduled clean-ups and or sanitation during the manufacturing shift
NOTE In industrial practice, the order and the number of coating steps may differ and may therefore deviate considerably from this scheme.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to insufficient or excessive cover of coating.
Dry coating should be carried out in accordance with the following:
There are some variations in industrial production for the frying process, in so far, that quick-frozen coated products are completely fried, and re-frozen later. For this case, alternative hazards and
80defects have to be described and not all statements in this section apply. In some regions it is common practice to manufacture raw coated fish products.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Pre-frying should be carried out in accordance with the following:
Potential hazards include, but are not limited to foreign material.
Potential defects include, but are not limited to:
Re-freezing should be carried out in accordance with the following:
81Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Packaging and labelling should be carried out in accordance with the following:
NOTE See 9.3.3, 9.5.4 and 9.3.1.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Storage of end products should be carried out in accordance with the following:
NOTE See 9.2.5.
Potential hazards are unlikely.
Potential defects include, but are not limited to thawing of frozen product.
During all transportation steps, deep-frozen conditions should be maintained at −18 °C with maximum fluctuation of ± 3 °C until final destination of product is reached.
Cleanliness and suitability of the transport vehicle to carry frozen food products should be examined.
Use of temperature recording devices with the shipment is recommended.
NOTE See also 4.7 and 16.
Coated molluscan shellfish should be manufactured from safe and wholesome molluscs.
Molluscan shellfish should be subject to regulation and controls of a competent authority that ensures safety for consumption.
Shellfish can be cooked or be raw prior to the coating process and should not contain significant defects.
83EXAMPLE Significant defects include sand, cuts, parasites or discoloration
NOTE 1 Annex C provides the application of HACCP principles and additional information for pre-requisite programmes.
NOTE 2 Annex D provides an example of a flow chart for coated molluscan shellfish processing.
All incoming raw materials should be subject to an examination for food safety hazards and defects based on the appropriate CODEX Alimentarius Commission Standard CODEX STAN 234-1999.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Molluscan shellfish should be processed in accordance with the following:
NOTE The use of temperature recording devices with the shipment is recommended.
NOTE See also 8.
For details on other ingredients of molluscan shellfish, refer to 11.4.1.2.
For details on packaging materials for molluscan shellfish, refer to 11.4.1.3.
For details on frozen storage of molluscan shellfish, refer to 11.4.2.1.
For details on other ingredients and packaging materials of molluscan shellfish, refer to 11.4.2.3.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Refrigerated storage of molluscan shellfish should be carried out in accordance with the following:
NOTE See 8.6.6.
For details on unpacking and wrapping of molluscan shellfish in refrigerated storage, refer to 11.4.4.
85Potential hazards include, but are not limited to microbiological growth.
Potential defects include, but are not limited to:
Thawing of frozen, coated molluscan shellfish should be carried out in accordance with the following:
Potential hazards include, but are not limited to contamination from dirty deglazing water.
Potential defects include, but are not limited to:
Deglazing should be carried out in accordance with the following:
For details on separation of individual molluscan shellfish for deglazing, refer to 11.4.6.
86For details on coating molluscan shellfish, refer to 11.4.7.
For details on wet coating molluscan shellfish, refer to 11.4.7.1.
For details on dry coating molluscan shellfish, refer to 11.4.7.2.
For details on pre-frying molluscan shellfish, refer to 11.4.8.
For details on re-freezing – final freezing molluscan shellfish, refer to 11.4.9.
For details on the packing and labelling of molluscan shellfish, refer to 11.4.10.
For details on the storage of the end product for molluscan shellfish, refer to 11.4.11.
For details on transport of the end product for molluscan shellfish, refer to 11.4.12.
Coated or breaded shrimp should be manufactured from good quality shrimp that have been subjected to sanitary conditions and processed under conditions that properly control food safety hazards.
Coated shrimp should be removed from their shells with the exception of the tail and with the alimentary canal or vein removed.
NOTE 1 See Annex C for the application of HACCP principles and additional information for pre-requisite programmes.
NOTE 2 See Annex D for an example of a flow chart for coated shrimp processing.
All incoming raw materials should be subject to an examination for food safety hazards and defects based on the appropriate CODEX Alimentarius Commission Standard CODEX STAN 234-1999.
NOTE See 13.
87Potential hazards include, but are not limited to sulphites.
Potential defects include, but are not limited to:
The reception of shrimp should be carried out in accordance with the following:
NOTE See 13.3.1
88For details on the presence of other ingredients in shrimp, refer to 11.4.1.2.
For details on the packaging material for shrimp, refer to 11.4.1.3.
For details on the frozen storage of shrimp, refer to 11.4.1.2.1 and 13.3.2.
For details on other ingredients and packaging material for shrimp, refer to 11.4.2.3.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Refrigerated storage of shrimp should be carried out in accordance with the following:
NOTE See 11.4.2.2.
For details on the unwrapping and unpacking of shrimp, refer to 11.4.4.
Potential hazards include, but are not limited to microbiological growth.
89Potential defects include, but are not limited to:
Thawing of frozen, coated shrimp should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Peeling, deveining and butterflying of shrimp should be carried out in accordance with the following:
NOTE See 4.5.
For details on the coating of shrimp, refer to 11.4.7.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Wet coating should be carried out in accordance with the following:
NOTE Bacterial toxin production is a possibility in batter mixes therefore usage times and temperatures should be set and cleaning schedules of equipment defined and maintained;
NOTE Batter that is too thin or thick may result in a coating and flesh ratio that does not meet specifications and regulatory requirements.
NOTE See 11.4.7.1.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Dry coating should be carried out in accordance with the following:
NOTE See 11.4.7.2.
For details on the pre-frying of shrimp, refer to 11.4.8.
92Fryers should be operated by trained staff.
Oil should be changed on a regular basis to avoid oxidative rancidity.
Oil temperatures should be controlled to avoid burning crumb or fire risk.
NOTE Whilst frying is necessary for tempura batter coatings, it may not always be used for crumb coating operations, although it may aid adhesion.
For details on the packaging and labelling of shrimp, refer to 11.4.10.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Blast freezing should be carried out quickly with the appropriate temperature and air flow parameters routinely monitored.
NOTE When the internal product temperature is between 0 °C and − 4 °C, quick blast freezing minimizes crystallization of the flesh and the moisture migration that will occur from the flesh to the coating.
Potential hazards include, but are not limited to microbiological growth.
Potential defects include, but are not limited to:
Casing of the frozen containers should be carried out quickly to prevent thawing and quality problems.
NOTE Texture changes of the shrimp flesh and moisture migration from the flesh to the coating can occur if casing is not carried out quickly.
For details on the frozen storage of the end product of shrimp, refer to 11.4.11.
For details on the transport of the end product of shrimp, refer to 11.4.12.
Salted fish and fish products and dried salted fish and fish products should be wholesome, well prepared and packaged so that they will be protected from contamination and remain attractive and safe to eat.
NOTE 1 See Annex C for the application of HACCP principles and additional information for pre-requisite programmes.
NOTE 2 See Annex D for an example of a flow chart for the processing of salted and dried salted fish.
NOTE 3 See 9.2 for general handling prior to processing.
General processes for salting should be carried out in accordance with the following:
Potential hazards are unlikely.
94Potential defects include, but are not limited to improper splitting.
Splitting, washing and rinsing should be carried out in accordance with the following:
NOTE It is important to cut the bone rather than to break it from the flesh.
For details on the filleting, skinning and trimming of fish for the drying and salting process, refer to 9.2.8.
For details on preparing round fish for drying and salting, refer to 9.2.2 to 9.2.7.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Nobbing should be carried out in accordance with the following:
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Gibbing should be carried out in accordance with the following:
Potential hazards include, but are not limited to chemical and physical contamination.
Potential defects include, but are not limited to incorrect composition.
Salt requirements should be in accordance with the following:
NOTE 1 The composition of salt differs according to the origin. Mine salt and solar salt of marine origin contain several other salts like calcium sulphate, magnesium sulphate and chloride as impurities. Vacuum processed and refined salt is almost pure sodium chloride.
96NOTE 2 Too much calcium may reduce the rate of salt penetration to an extent that spoilage may occur.
NOTE 3 Where present at too high a concentration, magnesium salts will give rise to unpleasant bitter flavours and may cause spoilage during the salting operation.
NOTE 4 Salt produced from marine sources may contain halophilic bacteria and mould which continue to live in the salt and dry salted fish and could contribute to spoilage.
NOTE 1 The use of very fine salt granules could result in the formation of clusters which are not favourable for ensuring the uniform distribution of salt on the fish.
NOTE 2 The use of very coarse salt granule could result in damage to the fish flesh during salting and may reduce the rate of maturation.
Potential hazards include, but are not limited to chemical and physical contamination.
Potential defects include, but are not limited to:
Salt handling should be carried out in accordance with the following:
Salted fish should be salt-matured, sound and wholesome.
In order to prevent the development of Clostridium botulinum:
Salting of fish either by brining, brine injection, wet-salting, dry-salting or pickling should be carried out with full understanding of their effects on the quality of the final product and should be done under strict hygienic condition and temperature control.
Salted fish should be kept at a temperature lower than 8 °C to minimise the occurrence of bacteria and mould.
In order to minimise microbial contamination of salted fish, previously used and or contaminated salt should be removed from the plant.
During the process of salting, the quality of the salt should be maintained by utilizing low temperatures and avoiding light and oxygen in an effort to reduce rancidity.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Brining should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Brine injection should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but is not limited to decomposition.
Wet-salting should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Dry-salting should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Pickling should be carried out in accordance with the following:
NOTE Pickling is primarily used for fatty fish. Under certain conditions dry salting of small fatty fish may be used.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Maturing should be carried out in accordance with the following:
NOTE 1 The length of this period will vary from a few weeks, to several months, depending on the specific product.
NOTE 2 Where the containers are to be held at lower temperatures, the maturing period will increase.
In general, reference should be made to 9.3.3 and 9.5.4 for sorting, drying, weighing, packaging, wrapping and labelling of salted fish.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Sorting should be carried out in accordance with the following:
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Drying should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
Potential defects are unlikely.
Weighing, wrapping, and packaging should be carried out in accordance with the following:
In general, reference should be made to 9.3.3 and 9.6 for labelling.
Potential hazards are unlikely.
Potential defects are unlikely.
Chill storage should be carried out in accordance with the following:
In general, reference should be made to 9.6 for packaging, labels and ingredients.
Shrimp frozen for further processing should be whole, head-off or de-headed or raw headless, peeled, peeled and de-veined, or cooked on board harvest or processing vessels or at on shore processing plants.
NOTE See Annex C for the application of HACCP principles and additional information for pre-requisite programmes.
Annex D provides an example of a flow chart for the processing of shrimp and prawns.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Raw fresh and frozen shrimp reception should be carried out in accordance with the following:
EXAMPLE These potential hazards include phytotoxins in sea caught shrimp (specifically for head on products) and potential antibiotics presence in aquaculture shrimp.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Frozen storage should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to decomposition.
Controlled thawing should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to decomposition.
Chilled storage should be carried out in accordance with the following:
106NOTE See 9.2.4
Potential hazards are unlikely.
Potential defects include, but are not limited to decomposition.
Shrimp should be selected for different quality grades according to specification requirements, and should be re-iced without delay.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to decomposition.
Size grading should be carried out in accordance with the following:
NOTE Mechanically graded shrimp can become trapped in the bars of the graders therefore regular inspection is required to prevent ‘carry over’ of old prawns and bacteriological contamination.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Addition of ingredients and use of additives should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological cross contamination.
Potential defects include, but are not limited to:
Full and partial peeling should be carried out in accordance with the following:
NOTE 1 This process applies mainly to warm water prawns and could be as simple as inspecting and preparing whole large prawns for freezing and down-grading blemished prawns for full peeling.
108NOTE 2 Peeling stages could include full peeling or partial peeling leaving tail swimmers intact.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Deveining should be carried out in accordance with the following:
NOTE This may be partially achieved with head-off, shell-on shrimp as well.
NOTE This operation is considered to be mechanical, though labour intensive.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Washing should be carried out in accordance with the following:
109Potential hazards include, but are not limited to:
Potential defects include, but are not limited to over-cooking.
The cooking process should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to presence of shell.
Peeling of cooked shrimp should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects are unlikely.
Cooling should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
The freezing process should be carried out in accordance with the following:
NOTE 1 Raw, whole or head-off shrimp may be block or plate frozen in purpose-designed cartons into which potable water is poured to form a solid block with protective ice.
111NOTE 2 Cooked and peeled Pandalus cold water prawns tend to be frozen through fluidised bed systems, whilst many of the warm water shrimp products are quick-frozen either on trays in blast freezers or in continuous belt freezers.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
NOTE 1 Ice block frozen shrimp is a form of glazing whereby frozen shrimp are dipped in chilled potable water and drained.
NOTE 2 A more sophisticated process is to pass frozen size graded shrimp under cold-water sprays on vibratory belts such that the shrimp pass at a steady rate to receive an even and calculable glaze cover.
NOTE See CODEX Alimentarius Commission, Methods for the determination of glaze.
In general, reference should be made to 9.5.4 and 9.6 for weighing, packing and labelling of all products.
Potential hazards include, but are not limited to sulphites.
112Potential defects include, but are not limited to:
Weighing, packing and labelling of products should be carried out in accordance with the following:
Potential hazards include, but are not limited to presence of metal.
Potential defects are unlikely.
Products should be metal detected in final pack through machines set to the highest sensitivity possible.
NOTE Large packs will be detected at a lower sensitivity than smaller packs so that consideration should be given to testing product prior to packing. However, unless potential re-contamination prior to packing can be eliminated, it is probably still better to check in-pack.
In general, reference should be made to 9.2.5 for frozen storage of end product.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Frozen storage of end product should be carried out in accordance with the following:
NOTE Fitting of a continuous recording thermometer is strongly recommended.
Fresh cephalopods should be handled at all times with great care and in such a way as to prevent contamination and inhibit the growth of micro-organisms as they are extremely perishable.
Cephalopods should not be exposed to direct sunlight or to the drying effects of winds, or any other harmful effects of the elements, but should be carefully cleaned and promptly cooled to 0 °C.
NOTE 1 This section applies to fresh and processed cephalopods including cuttlefish (Sepia and Sepiella), squid (Alloteuthis, Berryteuthis, Dosidicus, Ilex, Lolliguncula, Loligo, Loliolus, Nototodarus, Ommastrephes, Onychoteuthis, Rossia, Sepiola, Sepioteuthis, Symplectoteuthis and Todarodes) and octopuses (Octopus and Eledone) intended for human consumption.
NOTE 2 See Annex C for the application of HACCP principles and additional information for pre-requisite programmes.
NOTE 3 See Figure D.12 for an example of a flow chart for the processing of cephalopods.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Reception of cephalopods should be carried out in accordance with the following:
EXAMPLE TVBN
NOTE See 9 and the CODEX Alimentarius Commission Guidelines for Sensory Evaluation of Fish and Shellfish in Laboratories.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
In general, reference should be made to 9.2.4.
115Potential hazards include, but are not limited to heavy metals.
EXAMPLE Cadmium migration from the gut
Potential defects include, but are not limited to freezer-burn.
Frozen storage should be carried out in accordance with the following:
NOTE See 9.2.5.
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Controlled thawing should be carried out in accordance with the following:
NOTE See 9.2.6.
116Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Splitting, gutting and washing should be carried out in accordance with the following:
Potential hazards include, but are not limited to microbiological contamination.
Potential defects include, but are not limited to:
Skinning and trimming should be carried out in accordance with the following:
NOTE Enzymatic skinning or hot water techniques should have defined time or temperature parameters to prevent the growth of micro-organisms.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
The application of additives should be carried out in accordance with the following:
In general, reference should be made to 9.3.3 for labelling.
Potential hazards include, but are not limited to chemical or physical contamination from packaging.
118Potential defects include, but are not limited to:
Grading, packing and labelling should be carried out in accordance with the following:
Potential hazards include, but are not limited to parasites.
Potential defects include, but are not limited to:
Freezing should be carried out in accordance with the following:
In general, reference should be made to 9.4.1 and Annex A.
Consideration should be given to the potential hazards and defects associated with packaging, labelling and ingredients (see 9.6).
This section applies to the processing of heat processed sterilised canned fish, shellfish and other aquatic invertebrate products which have been packed in hermetically sealed containers and intended for human consumption.
NOTE 1 See Annex C for the application of HACCP principles and additional information for pre-requisite programmes.
NOTE 2 See Annex D for an example of a flow chart for the processing of fish, shellfish and other aquatic invertebrates.
General additions to a pre-requisite programme should be carried out in accordance with the following:
NOTE 1 See 4.
NOTE 2 See CODEX Alimentarius Commission Recommended International Code of Hygienic Practice for Low-Acid and Acidified Low- Acid Canned Food (CAC/RCP 23-1979).
The identity of the species and or the origin of fish intended for processing are known as biotoxins and are generally heat-stable.
The status of the area of origin of molluscan shellfish or other affected species intended for processing are known as phycotoxins and are are heat stable.
Good practices for the conservation and handling, from capture to heat processing, should be in place to prevent histamine production.
NOTE 1 Histamine is heat-stable, and so its toxicity remains practically intact in containers.
NOTE 2 The CODEX Alimentarius Commission adopted in its standards for some fish species maximum levels tolerated for histamine.
High sterilisation values should be employed to destroy Clostridium botulinum spores, in particular those from proteolytic strains.
The proliferation and contamination risks of botulinum should be limited during processing.
The high risk of botulinum should be controlled by any of the following:
NOTE 1 The botulism toxin is heat-sensitive. Botulism risk usually appears after an inadequate heat processing and inadequate container integrity. The heat processing effectiveness depends on the contamination level at the time of the treatment.
NOTE 2 Toxins from Staphylococcus aureus can be present in a highly contaminated raw material or can be produced by bacterial proliferation during processing. After canning, there is also the potential risk of post process contamination with Staphylococcus aureus if the warm wet containers are handled in an unsanitary manner. These toxins are heat-resistant, so should be taken into account in the hazard analysis.
121Care should be taken to avoid contamination of the product from components of the containers.
EXAMPLE Lead and chemical products: lubricants, sanitizers, detergents
Containers prior to filling may contain materials such as metal or glass fragments.
Potential defects are outlined in the essential quality, labelling and composition requirements described in the relevant CODEX Alimentarius Commission Standards. Where no CODEX Alimentarius Commission Standard exists, regard should be made to national regulations and or commercial specifications.
Processors may also refer to the Recommended International Code of Hygienic Practice for Low-Acid and Acidified Low-Acid Canned Foods (CAC/RCP 23-1979).
Potential hazards include, but are not limited to:
EXAMPLE DSP, PSP, scombrotoxin and heavy metals
Potential defects include, but are not limited to:
Where live shellfish (crustaceans) are received for canning processing, inspection should be carried out in order to discard dead or badly damaged animals.
NOTE See 9.2.2 and other relevant sections.
122Potential hazards include, but are not limited to subsequent microbiological contamination.
Potential defects include, but are not limited to tainting of the product.
Container, cover and packaging materials should be in accordance with the following:
NOTE See 9.6.1.
In general, reference should be made to 9.6.1.
In general, reference should be made to 9.2.4, 9.2.5 and 8.6.3.
123Potential hazards are unlikely.
Potential defects include, but are not limited to foreign matter.
Containers and packaging should be stored in accordance with the following:
NOTE See 9.6.2.
In general, reference should be made to 9.6.2.
Potential hazards are unlikely.
Potential defects include, but are not limited to foreign matter.
Unwrapping and unpacking should be carried out in accordance with the following:
In general, reference should be made to 9.2.6.
124Potential hazards include, but are not limited to:
EXAMPLE histamine
Potential defects include, but are not limited to:
EXAMPLE viscera, skin, scales
Where skinning of fish is performed by soaking in soda solution, an appropriate neutralisation should be carried out.
NOTE See 9.2.7 and 9.2.8.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to objectionable matter.
Preparation of molluscs and crustaceans should be carried out in accordance with the following:
NOTE Refer to 8.8.2.
Potential hazards include, but are not limited to:
EXAMPLE Polar components of oxidised oils
Potential defects include, but are not limited to:
Pre-cooking and other treatments should be carried out in accordance with the following:
NOTE The choice of method is usually strongly influenced by the nature of the treated material.
NOTE See CODEX Alimentarius Commission Standard for Named Vegetable Oils (CODEX STAN 210-1999), CODEX Alimentarius Commission Standard for Olive Oils and Olive Pomace Oils (CODEX STAN 33-1981) and CODEX Alimentarius Commission Standard for Fats and Oils not Covered by Individual Standards (CODEX STAN 19-1981).
NOTE The exception is for products which are packed when still hot.
Potential hazards include, but are not limited to microbiological and chemical contamination by the dip solution.
Potential defects include, but are not limited to:
The use of brine and other dips should be carried out in accordance with the following:
Potential defects include, but are not limited to:
Filling of containers should be carried out in accordance with the following:
NOTE A regular filling is important not only for economical reasons, but also because the heat penetration and the container integrity can be affected by excessive filling changes.
NOTE The necessary amount of headspace will depend partly on the nature of the contents;
Potential hazards include, but are not limited to contamination due to a bad seam.
Potential defects are unlikely.
Sealing of containers should be carried out in accordance with the following:
129NOTE Qualified personnel should conduct this operation.
NOTE 1 This is useful for deep containers or glass containers. It is difficult and hardly necessary to create a vacuum in shallow containers that have relatively large flexible covers.
NOTE 2 Excessive vacuum may cause the container to panel, particularly if the headspace is large, and may also cause contaminants to be sucked into the container if there is a slight imperfection in the seam.
Potential hazards include, but are not limited to subsequent contamination due to damaged containers.
Potential defects include, but are not limited to loss of traceability due to an incorrect coding.
130Coding of containers should be carried out in accordance with the following:
NOTE Manufacture details include: type of product, cannery where the canned fish or shellfish was produced, production date, etc.
NOTE Coding may sometimes be carried out after the cooling step.
Potential hazards include, but are not limited to:
Potential defects are unlikely.
Handling of containers after closure should be carried out in accordance with the following:
The Recommended International Code of Hygienic Practice for Low-Acid and Acidified Low-Acid Canned Foods (CAC/RCP 23-1979) provides detailed advice on heat processing.
Potential hazards include, but are not limited to survival of spores of Clostridium botulinum.
131Potential defects include, but are not limited to survival of micro-organisms responsible for decomposition.
Thermal processing should be carried out in accordance with the following:
NOTE When determining the sterilisation schedule, consideration should be given but not limited to microbial flora, dimensions and nature of the container, and product formulation. A sterilisation schedule is established for a certain product in a container of a given size.
NOTE Container size and type, retort installation and loading equipment and procedures should be considered.
NOTE See the Recommended International Code of Hygienic Practice for Low-Acid and Acidified Low-Acid Canned Foods (CAC/RCP 23-1979).
132NOTE The air pressure should be applied before the water temperature is raised.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Cooling should be carried out in accordance with the following:
133NOTE Efficiency of the treatment other than chlorination should be monitored and verified.
NOTE Rapid cooling of canned fish and shellfish avoids the formation of struvite crystals.
NOTE This assessment should be made as soon as practical after the canned fish and shellfish have been produced, so that if there are any faults due to failings on the part of cannery workers or canning equipment, these failings can be promptly corrected. Segregating and properly disposing of all defective units or lots that are unfit for human consumption should be ensured.
Potential hazards include, but are not limited to subsequent recontamination due to the damage of containers or an exposition to extreme conditions.
Potential defects include, but are not limited to incorrect labelling.
134Labelling, casing and storage of finished products should be carried out in accordance with the following:
NOTE Pallets of finished products should not be stacked excessively high and the forklift trucks used for the storage should be used in a proper manner.
NOTE See 9.3.3.
Potential hazards include, but are not limited to subsequent recontamination due to the damage of containers or an exposition to extreme conditions.
Potential defects are unlikely.
Transportation of finished products should be in accordance with the following:
NOTE Forklift trucks used during the loading and unloading should be used in a proper manner.
NOTE See section 16.
135Annex C provides the application of HACCP principles and additional information for prerequisite programmes.
NOTE Refer to the Recommended International Code of Practice-General Principles of Food Hygiene, Section VIII- Transportation, CAC/RCP 1-1969 and the Code of Hygienic Practice for the Transport of Food in Bulk and Semi-Packaged Food (CAC/RCP 47-2001).
During the transportation of fresh, frozen or refrigerated fish, shellfish and their products, steps should be taken to ensure that the chill or frozen temperature is maintained under controlled conditions and any increase in temperature of the product is minimised. In addition, appropriate measures should be applied to minimize damage to products and their packaging.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Transport of fresh, refrigerated and frozen products should be carried out in accordance with the following:
NOTE Load stabilizer devices are recommended.
NOTE The use of a recording thermometer is recommended.
136NOTE See 4.7
Reference should be made to the specific provisions laid down in the relevant sections of this Code.
Reference should be made to the specific provisions laid down in 15.
Transportation of all products should be carried out in accordance with the following:
Annex C provides the application of HACCP principles and additional information for prerequisite programmes.
Fish, shellfish and their products at retail should be received, handled, stored and displayed to consumers in a manner that minimizes potential food safety hazards and defects and maintains essential quality. Consistent with the HACCP and DAP approaches to food safety and quality, products should be purchased from known or approved sources under the control of competent health authorities that can verify HACCP controls. Retail operators should develop and use written purchase specifications designed to ensure food safety and desired quality levels. Retail operators should be responsible to maintain quality and safety of products.
Proper storage temperature after receipt is critical to maintain product safety and essential quality and chilled products should therefore be stored in a hygienic manner at temperatures ≤ 4 °C, MAP products at 3 °C or lower, while frozen products should be stored at temperatures ≤ −18 °C.
Preparation and packaging should be carried out in a manner consistent with the principles and recommendations found in 4 and relevant CODEX Alimentarius Commission Labelling Standards. Product in open full display should be protected from the environment by use of display covers. At all times, displayed seafood items should be held at temperatures and conditions that minimize the development of potential bacterial growth, toxins and other hazards, and loss of essential quality.
Consumer information at the point of purchase that inform consumers about storage, preparation procedures and potential risks of seafood products if mishandled or improperly prepared, is important to ensure that product safety and quality is maintained.
EXAMPLE Consumer information includes placards or brochures
A system of tracking the origin and codes of fish, shellfish and their products should be established to facilitate product recall or public health investigations in the event of the failure of preventive health protection processes and measures.
NOTE These systems exist for molluscan shellfish in some countries in the form of molluscan shellfish tagging requirements.
In general, reference should be made to, 8.6.2.1 and 9.2.2.1
In general, reference should be made to 8.6.2.2 and 9.2.2.2
Reception of fish, shellfish and their products at retail should be carried out in accordance with the following:
NOTE Products subject to filth, taint or contamination should be rejected.
138NOTE Determine that cooked, ready-to-eat product has not been exposed to raw product, juices or live molluscan shellfish and that raw molluscan shellfish has not been exposed to other raw fish or shellfish.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Reception of chilled products at retail should be carried out in accordance with the following:
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Chilled storage of products at retail should be carried out in accordance with the following:
NOTE This system could be based on first in, first out usage, production date or best before date on labels, and sensory quality of the lot, as appropriate.
Potential hazards are unlikely.
Potential defects include, but are not limited to:
Frozen storage of products at retail should be carried out in accordance with the following:
140Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Preparation and packaging of chilled seafood at retail should be carried out in accordance with the following:
NOTE See 9.3.3.
Potential hazards include, but are not limited to:
Potential hazards include, but are not limited to:
Preparation and packaging of frozen seafood should be carried out in accordance with the following:
NOTE See 9.3.3.
Potential hazards include, but are not limited to:
Potential defects include, but are not limited to:
Retail display of chilled seafood should be in accordance with the following:
NOTE A diagram of display is recommended to ensure that cross contamination does not occur.
142Potential hazards are unlikely.
Potential defects include, but are not limited to:
Retail display of frozen seafood should be in accordance with the following:
NOTE A recording thermometer is recommended.
(informative)
The parasites known to cause disease in humans and transmitted by fish or crustaceans are broadly classified as helminths or parasitic worms. These are commonly referred to as Nematodes, Cestodes and Trematodes. Fish can be parasitised by protozoans, but there are no records of fish protozoan disease being transmitted to man. Parasites have complex life cycles, involving one or more intermediate hosts and are generally passed to man through the consumption of raw, minimally processed or inadequately cooked products that contain the parasite infectious stage, causing food-borne disease. Freezing at −20 °C or below for 7 days or −35 °C for about 20 hours for fish intended for raw consumption will kill parasites. Processes such as brining or pickling may reduce the parasite hazard if the products are kept in the brine for a sufficient time but may not eliminate it. Candling, trimming belly flaps and physically removing the parasite cysts will also reduce the hazards but may not eliminate it.
Many species of nematodes are known to occur worldwide and some species of marine fish act as secondary hosts. Among the nematodes of most concern are Anisakis spp., Capillaria spp., Gnathostoma spp., and Pseudoteranova spp., which can be found in the liver, belly cavity and flesh of marine fish. An example of a nematode causing disease in man is Anisakis simplex; as the infective stage of the parasite is killed by heating (60 °C for 1 min) and by freezing (−20 °C for 24 hrs) in the fish core.
Cestodes are tapeworms and the species of most concern associated with the consumption of fish is Dibothriocephalus latus. This parasite occurs worldwide and both fresh and marine fish are intermediate hosts. Similar to other parasitic infections, the food-borne disease occurs through the consumption of raw or under-processed fish. Similar freezing and cooking temperatures as applied to nematodes will inactivate the infective stages of this parasite.
Fish-borne trematode (flatworm) infections are major public health problems that occur endemically in about 20 countries around the world. The most important species with respect to the numbers of people infected belong to the genera Clonorchis and Ophisthorchis (liver flukes), Paragonimus (lung flukes), and to a lesser extent Heterophyes and Echinochasmus (intestinal flukes). The most important definitive host of these trematodes is man or other mammals. Freshwater fish are the second intermediate host in the life cycles of Clonorchis and Ophistorchis, and freshwater crustaceans in the case of Paragonimius. Food-borne infections take place through the consumption of raw, undercooked or otherwise under-processed products containing the infective stages of these parasites. Freezing fish at −20 °C for 7 days or at −35 °C for 24 hrs will kill the infective stages of these parasites.
145The level of contamination of fish at the time of capture will depend on the environment and the bacteriological quality of the water in which fish are harvested. Many factors will influence the microflora of finfish, the more important being water temperature, salt content, proximity of harvesting areas to human habitations, quantity and origin of food consumed by fish, and method of harvesting. The edible muscle tissue of finfish is normally sterile at the time of capture and bacteria are usually present on the skin, gills and in the intestinal tract.
There are two broad groups of bacteria of public health importance that may contaminate products at the time of capture - those that are normally or incidentally present in the aquatic environment, referred to as the indigenous microflora, and those introduced through environmental contamination by domestic and or industrial wastes. Examples of indigenous bacteria, which may pose a health hazard, are Aeromonas hydrophyla, Clostridium botulinum, Vibrio parahaemolyticus, Vibrio cholerae, Vibrio vulnificus, and Listeria monocytogenes. Non-indigenous bacteria of public health significance include members of the Enterobacteriaceae, such as Salmonella spp., Shigella spp., and Escherichia coli. Other species that cause food-borne illness and which have been isolated occasionally from fish are Edwardsiella tarda, Pleisomonas shigeloides and Yersinia enterocolitica. Staphyloccocus aureus may also appear and may produce heat resistant toxins.
Indigenous pathogenic bacteria, when present on fresh fish, are usually found in fairly low numbers, and where products are adequately cooked prior to consumption, food safety hazards are insignificant. During storage, indigenous spoilage bacteria will outgrow indigenous pathogenic bacteria, thus fish will spoil before becoming toxic and will be rejected by consumers. Hazards from these pathogens can be controlled by heating seafood sufficiently to kill the bacteria, holding fish at chilled temperatures and avoiding post- process cross-contamination.
Vibrio species are common in coastal and estuarine environments and populations can depend on water depth and tidal levels. They are particularly prevalent in warm tropical waters and can be found in temperate zones during summer months. Vibrio species are also natural contaminants of brackish water tropical environments and will be present on farmed fish from these zones. Hazards from Vibrio spp. associated with finfish can be controlled by thorough cooking and preventing cross-contamination of cooked products. Health risks can also be reduced by rapidly chilling products after harvest, thus reducing the possibility of proliferation of these organisms. Certain strains of Vibrio parahaemolyticus can be pathogenic.
Molluscan shellfish harvested from inshore waters that are contaminated by human or animal faeces may harbour viruses that are pathogenic to man. Enteric viruses that have been implicated in seafood-associated illness are the hepatitis A virus, caliciviruses, astroviruses and the Norwalk virus. The latter three are often referred to as small round structured viruses. All of the seafood-borne viruses causing illness are transmitted by the faecal-oral cycle and most viral gastroenteritis outbreaks have been associated with eating contaminated shellfish, particularly raw oysters.
Generally viruses are species specific and will not grow or multiply in foods or anywhere outside the host cell. There is no reliable marker for indicating presence of the virus in shellfish harvesting waters. Seafood-borne viruses are difficult to detect, requiring relatively sophisticated molecular methods to identify the virus.
Occurrence of viral gastro-enteritis can be minimized by controlling sewage contamination of shellfish farming areas and pre-harvest monitoring of shellfish and growing waters as well as controlling other sources of contamination during processing. Depuration and relaying are alternative strategies but longer periods are required for shellfish to purge themselves clean of viral contamination than for bacteria. Thermal processing (85 °C to 90 °C for 1½ min) will destroy viruses in shellfish.
146There are a number of important biotoxins to consider. Around 400 poisonous fish species exist and, by definition, the substances responsible for the toxicity of these species are biotoxins. The poison is usually limited to some organs, or is restricted to some periods during the year.
For some fish, the toxins are present in the blood and are called ichtyohaemotoxin. The involved species are eels from the Adriatic, the moray eels, and the lampreys. In other species, the toxins are spread all over the tissues (flesh, viscera, skin) and are called ichtyosarcotoxins. The tetrodotoxic species responsible for several poisonings, often lethal, are in this category.
In general these toxins are known to be heat-stable and the only possible control measure is to check the identity of the used species.
And the other important toxin to consider is ciguatoxin, which can be found in a wide variety of mainly carnivorous fish inhabiting shallow waters in or near tropical and subtropical coral reefs. The source of this toxin is dinoflagellates and over 400 species of tropical fish have been implicated in intoxication. The toxin is known to be heat stable. There is still much to be learnt about this toxin and the only control measure that can reasonably be taken is to avoid marketing fish that have a known consistent record of toxicity.
Paralytic Shellfish Poison (PSP), Diarrhetic Shellfish Poison (DSP), Neurotoxic Shellfish Poison (NSP), and Amnesic Shellfish Poison (ASP) complex are produced by phytoplankton. They concentrate in bivalve molluscan shellfish which filter the phytoplankton from the water, and also may concentrate in some fish and crustacea.
Generally, the toxins remain toxic through thermal processing so the knowledge of the species identity and or origin of fish or shellfish intended for processing is important.
Fish mainly belonging to the family Tetradontidea (“puffer fishes–) may accumulate this toxin which is responsible for several poisonings, often lethal. The toxin is generally found in the fish liver, roe and guts, and less frequent in the flesh. Differently from most other fish biotoxins that accumulate in the live fish or shellfish, algae do not produce this toxin. The mechanism of toxin production is still not clear, however, apparently there are often indications of the involvement of symbiotic bacteria.
Scombroid intoxication sometimes referred to as histamine poisoning, results from eating fish that have been incorrectly chilled after harvesting. Scombrotoxin is attributed mainly to Enterobacteriaceae which can produce high levels of histamine and other biogenic amines in the fish muscle when products are not immediately chilled after catching. The main susceptible fish are the scombroids such as tuna, mackerel, and bonito, although it can be found in other fish families such as Clupeidae. The intoxication is rarely fatal and symptoms are usually mild. Rapid refrigeration after catching and a high standard of handling during processing should prevent the development of the toxin. The toxin is not inactivated by normal heat processing. In addition, fish may contain toxic levels of histamine without exhibiting any of the usual sensory parameters characteristic of spoilage.
147Fish may be harvested from coastal zones and inland habitats that are exposed to varying amounts of environmental contaminants. Of greatest concern are fish harvested from coastal and estuarine areas rather than fish harvested from the open seas. Chemicals, organochloric compounds and heavy metals may accumulate in products that can cause public health problems. Veterinary drug residues can occur in aquaculture products when correct withdrawal times are not followed or when the sale and use of these compounds are not controlled. Fish can also be contaminated with chemicals such as diesel oil, when incorrectly handled and detergents or disinfectants when not properly rinsed out.
These can include material such as metal or glass fragments, shell, bones, etc.
148(informative)
A typical cleaning and disinfecting process may involve as many as seven separate steps:
NOTE This involves steps such as removal of all fish, shellfish and their products from area, protection of sensitive components and packaging materials from water, removal by hand or squeegee of fish scraps, etc.
(normative)
The HACCP System consists of seven principles (see Figure C.1):
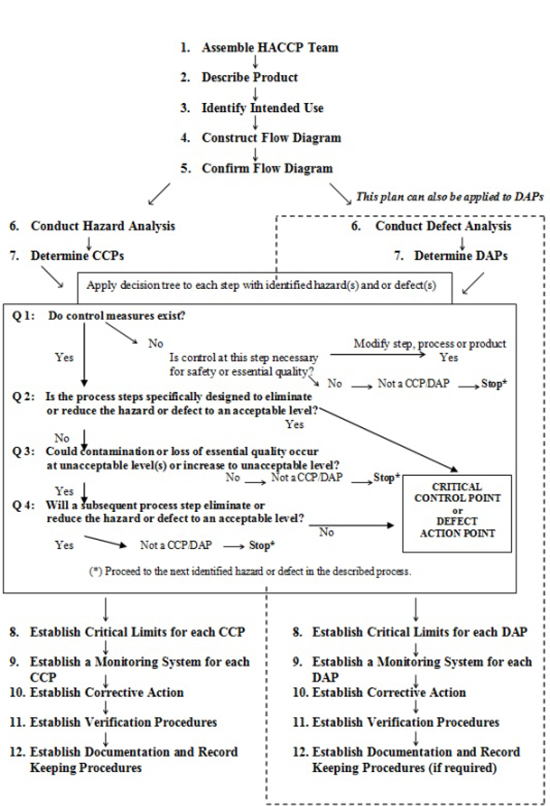
Figure C.1 — Summary of the implementation of a HACCP and defect analysis
These principles have to be followed when considering a HACCP plan.
HACCP is an important management tool, which can be used by operators for ensuring safe, efficient processing. It must also be recognised that personnel training is essential in order for HACCP to be effective. In following HACCP principles, users are requested to list all of the hazards that may be reasonably expected to occur for each type of product at each step or procedure in the process from point of harvest, during unloading, transport, storage or during processing, as appropriate to the process defined. It is important that HACCP principles be considered on a specific basis to reflect the risks of the operation.
151Since the Code is intended to cover not only those hazards associated with safety but to include other aspects of production including the essential product quality, composition and labelling provisions as described in product standards developed by the CODEX Alimentarius Commission, it includes both the CCPs and defect action points (DAP). The HACCP principles may be applied to the determination of a DAP, with quality instead of safety parameters being considered at the various steps.
Each aquaculture, molluscan shellfish, shellfish and fish facility should ensure that the provisions of the appropriate CODEX Alimentarius Commission standards are met. To accomplish this, each facility should implement a food safety management system based on HACCP principles and should at least consider a similar approach to defects, both of which are described in this code. Prior to the application of HACCP to any segment of the growing, handling and processing chain, that segment must be supported by a pre-requisite programme based on good hygienic practice (see 4). It should be noted that parts of the pre-requisite programme may be classified as a CCP or DAP within a particular process.
The established food management system should indicate responsibility, authority and the interrelationships of all personnel who manage, perform and verify work affecting the performance of such systems. The collection, collation and evaluation of scientific and technical data should be carried out by a multi-disciplinary team. Ideally, a team should consist of people with the appropriate level of expertise together with those having a detailed knowledge of the process and product under review. The personnel comprising the team should include the processing facility manager, a microbiologist, a quality assurance or quality control specialist, and others such as buyers, operators, and other personnel as necessary. For small-scale operations, it may not be possible to establish such a team and therefore external advice should be sought.
The scope of the HACCP plan should be identified and should describe which segments of the food chain are involved and the general classes of hazards to be addressed.
The design of this programme should identify critical control points in the operation where the processing facility or product will be controlled, the specification or standard to be met, the monitoring frequency and sampling plan used at the critical control point, the monitoring system used to record the results of these inspections and any corrective action when required. A record for each critical control point that demonstrates that the monitoring procedures and corrective actions are being followed should be provided. The records should be maintained as verification and evidence of the plant’s quality assurance programme. Similar records and procedures may be applied to DAPs with the necessary degree of record keeping. A method to identify, describe and locate the records associated with HACCP programmes should be established as part of the HACCP programme.
Verification activities include the application of methods; procedures (review/audit) and tests in addition to those used in monitoring to determine:
EXAMPLE Audit or review
The implementation of HACCP principles is better identified in the Logic Sequence for implementation of HACCP (see Figure C.1).
152In order to gain a greater understanding and knowledge of the product under review, a thorough product description evaluation should be carried out. This exercise will facilitate in the identification of potential hazards or defects. Table C.1 provides an example of the type of information used in describing a product.
| Product name(s) | Objective | Example |
| Identify the species and method of processing. | Canned tuna in salted water | |
| Source of raw material | Describe the origin of the fish | Yellow fin tuna caught by purse seine in the Gulf of Guinea
Whole brine frozen |
| Important final product characteristics | List characteristics that affect product safety and essential quality, especially those that influence microbial flora. | Compliance with CODEX Alimentarius Commission Standard Canned Tuna and Bonito; ‘low-acid’ food; can seal integrity. |
| Ingredients | List every substance added during processing. Only ingredients approved by the national competent authority may be used. | water, salt |
| Packaging | List all packaging materials. Only materials approved by the national competent authority may be used. | Container in coated chromium steel, capacity : 212 ml, total net weight : 185 g, fish weight : 150 g Traditional opening |
| How the end product is to be used | State how the final product is to be prepared for serving, especially whether it is ready to eat. | Ready to eat |
| Shelf life (if applicable) | State the date when the product can be expected to begin to deteriorate if stored | 3 years |
| Where the product will be sold | Indicate the intended market. This information will facilitate compliance with target market regulations and standards. | Domestic retail market. |
| Special labelling instructions | List all instructions for safe storage and preparation | “Best before the date shown on label” |
| Special distribution control | List all instructions for safe product distribution. | None |
For Hazard and Defect Analysis, it is necessary to carefully examine both the product and the process and produce a flow diagram(s). Any flow diagram should be as simple as possible. Each step in the process, including process delays from the selection of raw materials through to the processing, distribution, sale and customer handling, should be clearly outlined in sequence with sufficient technical data to avoid ambiguity. If a process is too complex to be easily represented by a single flow diagram, then it can be sub-divided into constituent parts, provided the relationship between each of the parts is clearly defined. It is helpful to number and label each processing step for ease of reference. An accurate and properly constructed flow diagram will provide the multi-disciplinary team with a clear vision of the process sequence. Once CCPs and DAPs have been identified they can be incorporated into the flow diagram specific for each processing facility. Figure D.1 represents an example of a flow diagram for a canned tuna fish processing line.
The purposes of hazard analysis are to identify all such food safety hazards at each step, to determine their significance and to assess whether control measures for those hazards are available at each step. Defect analysis serves the same purpose for potential quality defects.
153It cannot be stressed enough that where practical and feasible each individual facility should gather sound scientific and technical data relevant to the businesses for each step, from primary production, processing, manufacture, storage and distribution until the point of consumption. The assembly and nature of this information should be such to ensure that the multi-disciplinary team is able to identify and list, at each step of the process, all of the hazards that may reasonably likely to occur and defects that, in the absence of control measure(s), may likely result in the production of an unacceptable food. Potential hazards, which have been known to be associated with fresh fish and shellfish, are described in Annex A. Table C.2 summarises possible pre-harvest and harvest safety hazards in incoming fish and shellfish and Table C.3 summarises possible safety hazards introduced in the post harvest and further processing of fish and shellfish.
It is important to identify potential hazards and defects in the operation from the point of view of plant construction, equipment used in the plant and hygienic practices, including those which may be associated with the use of ice and water. This is covered by the pre-requisite programme and is used to denote hazards that are common to almost any point in the process.
NOTE 1 For biological hazards, environmental factors (for example: temperature, oxygen availability, pH and Aw) play a major role in their activity and growth, therefore the type of processing the fish or shellfish will undergo, and its subsequent storage, will determine their risk to human health and inclusion in a food safety management plan. In addition, some hazards may show a certain degree of overlap between the two levels of operation through their existence and manifestation into the water supply.
NOTE 2 For hazards relating to specific products see the relevant processing section.
| Biological | Chemical | Physical | |||
|---|---|---|---|---|---|
| Parasites: | Parasites of public health significance: Trematodes, Nematodes, Cestodes |
Chemicals: | Pesticides, herbicides, algicides, fungicides, anti-oxidants (added in feeds) |
Foreign Matter | fish hooks |
| Pathogenic bacteria: | Salmonella, Shigella, E. coli, Vibrio cholerae, Vibrio parahaemolyticus, Vibrio vulnificus |
Veterinary drug residues: | Antibiotics, growth promoters (hormones), other veterinary drugs and feed additives |
||
| Enteric Viruses: | Norwalk virus | Heavy metals: | Metals leached from marine sediments and soil, from industrial wastes, from sewage or animal manures | ||
| Biotoxins: | Biotoxins, Scombrotoxin |
||||
| Miscellaneous: | Petroleum | ||||
| Biological | Chemical | Physical | |||
|---|---|---|---|---|---|
| Pathogenic bacteria: | Listeria monocytogenes, Clostridium botulinum, Staphylococcus aureus |
Chemicals: | Disinfectants, sanitizers or lubricants (misapplication) | Foreign matter | Metal fragments; hard or sharp objects |
| Enteric Viruses: | Hepatitis A, Rotavirus | Disinfectants, sanitizers or lubricants (non-approved) | |||
| Biotoxins: | Scombrotoxin, Staph. Enterotoxin, botulinum toxin |
||||
| Ingredients and additives: | Misapplication and non-approved | ||||
For the example on canned tuna developed in this section, the essential potential hazards as indicated in table C.4 can be identified.
| In raw materials (frozen tuna) | During processing or storage or transportation | |
|---|---|---|
| Biological | Presence of C.botulinum | Contamination by C. botulinum |
| Presence of scombrotoxin | Growth of C. botulinum | |
| Survival of spores of C. botulinum, | ||
| Contamination and growth of Staphylococcus aureus | ||
| Recontamination after heat processing | ||
| Production of scombrotoxin during processing, | ||
| Production of staphylotoxin | ||
| Chemical | Presence of heavy metals | Recontamination by metals coming from the cans |
| Recontamination by cleaning agents, brine, mechanical grease | ||
| Physical | Presence of foreign material | Recontamination during processing (pieces of knives, by the cans, etc) |
For the example on canned tuna developed in this section, the potential defects can be identified as seen in Table C.5.
| In raw materials (frozen tuna) | During processing or storage or transportation | |
|---|---|---|
| Biological | Decomposition | Decomposition, survival of micro-organisms responsible for decomposition, etc |
| Chemical | Oxidation during storage, etc | |
| Physical | Objectionable matters (viscera, scales, skin, etc), formation of struvite crystals, container defects (panelled container, etc) | |
| Others | Species substitution | Abnormal flavours, incorrect weight, incorrect coding, incorrect labelling |
It is equally important to consider, naturally occurring food safety hazards in the environment from which fish or shellfish are harvested. In general, risks to consumer health from seafood captured in unpolluted marine environments are low, provided these products are handled in line with principles of Good Manufacturing Practice. However, as with all foods, there are some health risks associated with the consumption of certain products, which may be increased when the catch is mishandled after harvest. Fish from some marine environments, such as tropical reef fish, can pose a consumer risk from natural marine toxins, such as ciguatera. The risk of adverse health effects from certain hazards might be increased under certain circumstances in products from aquaculture when compared with fish and crustacean from the marine environment. The risks of food borne disease associated with products from aquaculture are related to inland and coastal ecosystems, where the potential of environmental contamination is greater when compared to capture fisheries. In some parts of the world, where fish or shellfish are consumed either raw or partially cooked, there is an increased risk of food borne parasitic or bacterial disease. In order to perform a hazard analysis as part of the process of developing a HACCP plan, processors must have scientific information on potential hazards associated with raw material and products for further processing.
One of the most important activities, which must be performed in a processing facility as part of the food safety management system is to determine if an identified hazard or defect is significant. The two primary factors that determine whether a hazard or defect is significant for HACCP purposes are probability of occurrence of an adverse health effect and the severity of the effect. A hazard that has a high severity of effect, such as death from Clostridium botulinum toxin, may impose a socially unacceptable risk at very low probability of occurrence, and thus warrant the application of HACCP controls (i.e., be a significant hazard for purposes of HACCP). Thus, in the processed canned tuna, Clostridium botulinum should be considered a significant hazard to be controlled through the application of a validated thermal process schedule. On the other hand, a hazard with a relatively low severity, such as mild gastroenteritis, might not warrant the HACCP controls at the same very low probability of occurrence, and thus not be significant for purposes of HACCP.
Information gathered during the product description exercise (see C.3.2) could also help facilitate the determination of significance since the likelihood of occurrence of hazard or defect can be affected by factors such as how the consumer will likely use the product (such as to be consumed or cooked raw); the types of consumers who will likely consume it (such as immuno-compromised, elderly, children, etc.) and the method of storage and distribution (such as refrigerated or frozen).
Once significant hazard and defects have been identified, consideration needs to be given to assess their potential to be introduced or controlled at each step of the process. The use of a flow diagram (see C.3.3) is beneficial for this purpose. Control measures must be considered for significant hazard(s) or defect(s) associated with each step with the aim of eliminating its possible occurrence or to reduce it to an acceptable level. A hazard or defect may be controlled by more than one control measure. For illustrative purposes, Tables C.6 and C.7 demonstrate an approach to listing significant hazards and defects and the related control measures for the processing step Heat Processing.
156| Processing step | Potential hazard | Is the potential hazard significant? | Justification | Control measures |
|---|---|---|---|---|
| 12. Heat processing | C. botulinum viable spores | Yes | An insufficient heat processing may result in survival of C. botulinum spores and therefore, possibility of toxin production. A product must be commercially sterile | Ensure adequate heat applied for proper time at retort |
| Processing step | Potential defect | Is the potential defect significant? | Justification | Control measures |
|---|---|---|---|---|
| 2. Storage of frozen | Persistent and distinct objectionable odours or flavours indicative of rancidity | Yes | Product does not meet quality or customer requirements | Controlled temperature in the storage premises |
| Stock management procedure | ||||
| Maintenance procedure of the refrigeration system | ||||
| Personnel training and qualification |
A thorough and concise determination of CCPs and DAPs in a process is important in ensuring food safety and compliance with elements related to essential quality, composition and labelling provisions of the appropriate CODEX Alimentarius Commission standard. The CODEX Alimentarius Commission decision tree (Figure C.1, step 7) is a tool which can be applied to the determination of CCPs and a similar approach may be used for DAPs. Using this decision tree, a significant hazard or defect at a step can be assessed through a logical sequence of questions. Where CCPs and DAPs have been identified at a step, that point in the process must be controlled to prevent, reduce or eliminate the likely occurrence of the hazard or defect to an acceptable level. For illustrative purposes, an example of the application of the CODEX Alimentarius Commission decision tree to a hazard and defect using the canned tuna fish processing line, are shown in Tables C.7 and C.8, respectively.
157| Processing step N° 12 Heat processing |
Application of CODEX Alimentarius Commission Decision Tree | ||||
|---|---|---|---|---|---|
| Potential hazards | Control measures | ||||
| C. botulinum viable spores | Ensure adequate heat applied for proper time at retort | Q1: Do control measures exist? If yes – go to Q2. If no – consider whether control measures are available or necessary within the process. Proceed to next identified hazard. |
Q2: Is the step specifically designed to eliminate or reduce the likely occurrence of C. botulinum to an acceptable level? If yes – this step is a CCP. If no – go to Q3. |
Q3: Could contamination occur in excess of acceptable levels or could this increase to unacceptable levels? If yes – go to Q4. If no – not a CCP. |
Q4: Will a subsequent step eliminate or reduce the hazard to an acceptable level? If yes – not a CCP. If no – CCP. What about consideration of a previous step? |
| A: Yes: a heat processing procedure (schedule, method) is clearly defined. | A: Yes, this step was specifically designed to eliminate spores. | ||||
| Decision: Processing step N°12 « Heat processing » is a Critical Control Point | |||||
| Processing step N° 2 Storage of frozen tuna |
Application of CODEX Decision Tree | ||||
|---|---|---|---|---|---|
| Potential Defects | Control Measures | ||||
| Persistent and distinct objectionable odours or flavours indicative of rancidity | Controlled temperature in storage premises.
Stock management procedure. |
||||
Q1 : Do control measures exist? If yes – go to Q2. If no – consider whether control measures are available or necessary within the process. Proceed to next identified hazard. |
Q2: Is the step specifically designed to eliminate or reduce the likely occurrence of rancidity to an acceptable level? If yes – this step is a DAP. If no – go to Q3. |
Q3: Could rancidity occur in excess of acceptable levels or could it increase to unacceptable levels? If yes – go to Q4. If no – not a DAP. |
Q4: Will a subsequent step eliminate rancidity or reduce its likely occurrence to acceptable level? If yes – not a DAP. If no – DAP. What about consideration of a previous step? |
||
| A: Yes, the storage temperature is controlled, procedures exist | A: No | A : Yes, if the storage time is too long and or the storage temperature is too high | A : No | ||
| Decision: Processing step N° 2 « Storage of frozen tuna » is a Defect Action Point | |||||
For each CCP and DAP, critical limits for the control of the hazard or defect must be specified. For any given hazard or defect, it may be necessary to have more than one critical limit designated for each control measure. The establishment of critical limits should be based on scientific evidence and validated by appropriate technical experts to ensure its effectiveness in controlling the hazard or defect to the determined level. Table C.9 illustrates critical limits for a CCP and a DAP using a canned tuna fish processing line as an example.
Any monitoring system developed by the multi-disciplinary team should be designed to detect loss of control at a CCP or DAP relative to its critical limit. The monitoring activity of a CCP or DAP should be documented in a concise fashion providing details regarding the individual responsible for the observation or measurement, the methodology used, the parameter(s) being monitored and the frequency of the inspections. The complexity of the monitoring procedure should also be carefully considered. Considerations include optimising the number of individuals performing the measurement and selection of appropriate methods, which will produce rapid results (for example: time, temperature, pH). For CCPs, records of monitoring should be acknowledged and dated by a responsible person for verification.
Since each process is unique for each product, it is possible only to present, for illustrative purposes, an example of a monitoring approach for a CCP and DAP using the canned tuna fish processing line. This example is shown in Table C.9.
An effective HACCP or DAP plan is anticipatory by nature and it is recognised that corrective action may be necessary from time to time. A documented corrective action programme should be established to deal with instances where the critical limit has been exceeded and loss of control has occurred at a CCP or DAP. The goal of this plan is to ensure that comprehensive and specific controls are in place and can be implemented to prevent the affected lot(s) from reaching the consumer. For example, fish and shellfish should be held and rejected if they are known to contain harmful substances and or defects which would not be eliminated or reduced to an acceptable level by normal procedures of sorting or preparation. Of equal importance, is an assessment by plant management and other appropriate personnel to determine the underlying reason(s) why control was lost. For the latter, a modification to HACCP and DAP plans may be necessary. A record of investigation results and actions taken should be documented by a responsible person for each instance where loss of control occurred at a CCP or DAP. The record should demonstrate that control of the process has been re-established, that appropriate product disposition has occurred and that preventative action has been initiated. An example of a corrective action approach for a CCP and DAP using a canned tuna fish processing line is illustrated in Table C.9.
A processing facility should establish a verification procedure carried out by qualified individuals, to periodically assess if the HACCP and DAP plans are adequate, implemented and working properly. This step will help determine if CCPs and DAPs are under control. Examples of verification activities include: validation of all components of the HACCP plan including: a paper review of HACCP system, its procedures and records; review of corrective actions and product disposition actions when critical limits are not met and validation of established critical limits. The latter is particularly important when an unexplained system failure has occurred, when a significant change to the process, product or packaging is planned or when new hazards or defects have been identified. Observation, measurement and inspection activities within the processing facility should also be incorporated as a part of the verification procedure, where applicable. Verification activities should be carried out by qualified competent individuals. The verification frequency of the HACCP and DAP plans should be sufficient to provide assurance that their design and implementation will prevent food safety problems as well as issues associated with essential quality, composition and labelling provisions
159of the appropriate CODEX standard to enable problems to be detected and dealt with in a timely manner. For illustration purposes, an example of a verification procedure approach for a CCP and DAP using the canned tuna fish processing line is shown in Table C.9.
Documentation may include Hazard Analysis, CCP determination, critical limit determination, and procedures for monitoring, corrective action and verification.
A current, accurate and concise record keeping system will greatly enhance the effectiveness of a HACCP programme and facilitate in the verification process. Examples of the elements of a HACCP plan that should be documented have been provided in this section for illustrative purposes. Inspection and corrective action records should be practical and collect all the appropriate data necessary to demonstrate “real-time” control or deviation control of a CCP. Records are recommended but not required for a DAP except where a loss of control occurred. For illustration purposes, an example of a record keeping approach for a CCP and DAP using the canned tuna fish processing line is shown in Table C.9.
| CCP | ||||
|---|---|---|---|---|
Processing step No. 12 : Heat processing Hazard: Clostridium botulinum viable spores |
||||
| Critical Limit | Monitoring Procedure | Corrective Action | Records | Verification |
| Those specific parameters associated with heat processing. | Who: Qualified person assigned to heat processing What: All parameters Frequency: every batch How: Checks of sterilisation schedule and other factors |
Who: qualified personnel What: Personnel retraining New heat processing or batch destruction Corrective maintenance of equipment Hold product until safety can be evaluated. Who: Appropriate trained personnel |
Monitoring records, corrective action records, product evaluation records, calibration records, validation records, audit records, HACCP plan review record |
Validation, finished product evaluation, internal audit, review of records, calibration of machinery (may be a prerequisite), review of HACCP plan, external audit |
| DAP | ||||
|---|---|---|---|---|
Processing step No. 2 : Storage of frozen tuna Defect: Persistent and distinct objectionable odours or flavours indicative of rancidity |
||||
| Critical Limit: | Monitoring Procedure | Corrective Action | Records | Verification |
Number of rancid sample units cannot exceed acceptance number of established sampling plan. Storage temperature and time. |
Who: Appropriate trained personnel How: Organoleptic examination Chemical tests Checking of the storage premise temperature Checking of stock forms What: fish quality and acceptability based on product CODEX standard Frequency: as required |
What: Application of an intensified monitoring According to the results of this intensified inspection, immediate processing, sorting or reject of frozen tuna exceeding the critical limits. Adjust storage temperature. Personnel retraining Who: Appropriate trained personnel |
Analysis results Stock forms Temperature records |
On-site audit Review of monitoring and corrective action reports |
Upon completion of all the steps for the development of HACCP and DAP plans as outlined in Figure C.1, a full review of all components should be conducted. The purpose of these reviews is to verify that the plans are capable of meeting their objectives.
Annex C has demonstrated the principles of HACCP and how they should be applied to a process to ensure safe product. The same principles can be used to determine the points in a process where it is necessary to control defects. Since every facility and each processing line is different it is possible within this Code only to demonstrate the types of potential hazards and defects that must be considered. Furthermore, because of the nature of the significance of hazards and defects it is not possible to categorically determine which steps in a process will be CCPs and or DAPs without actually assessing the process, the objectives of the process, its environment and expected outcomes. The example of the canned tuna processing line is intended to illustrate how to apply the principles, given the outcome of a commercially sterile product, and why a HACCP and DAP plan will be unique to each operation.
The remaining sections in the Code concentrate on aquaculture and molluscan shellfish production and to the handling and processing of fish, shellfish and their products and attempt to illustrate the potential hazards and defects at the various stages in a wide range of processes. In developing a HACCP or DAP plan it will be necessary to consult 4 and Annex C before turning to the appropriate processing section for specific advice. It should also be noted that 9 refers to processing of fresh, frozen and minced fish and will provide useful guidance for most of the other processing operations.
161(informative)
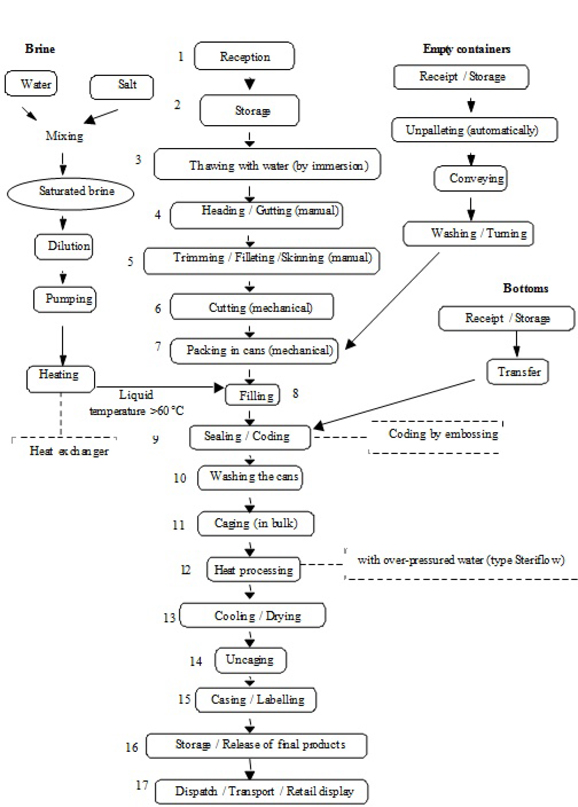
Figure D.1 — Example of a flow diagram for a processing line of canned tuna fish in brine
162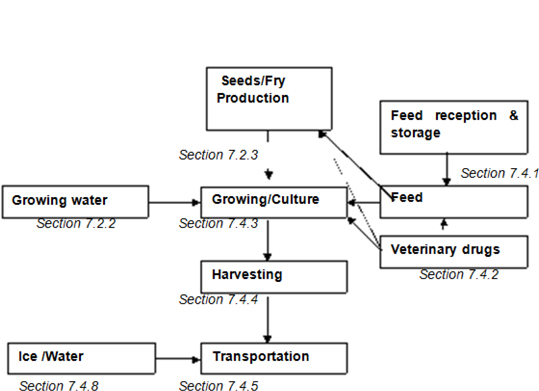
Figure D.2 — Example of a flow chart for aquaculture production
163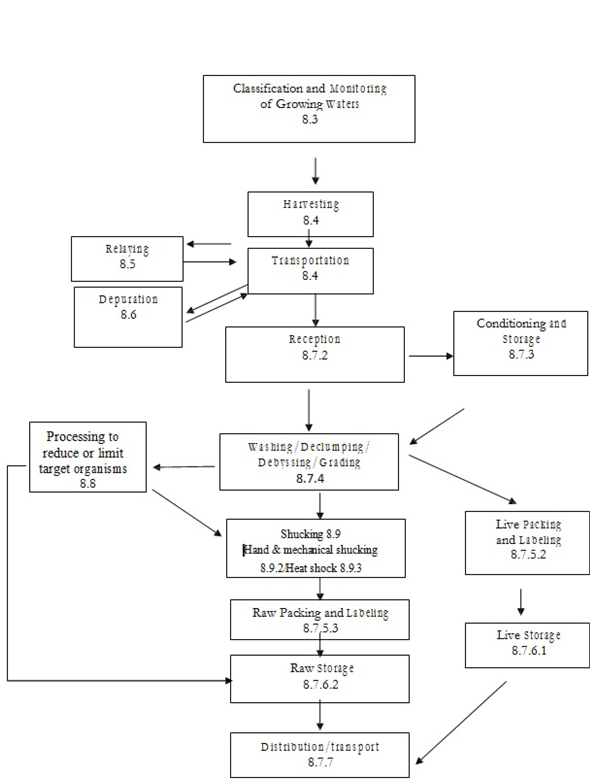
Figure D.3 — Example of a simplified flow diagram for production of live and raw bivalve molluscs
164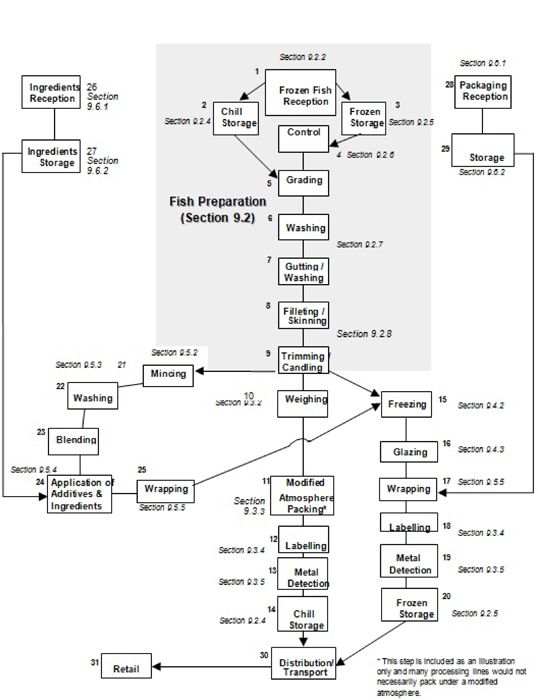
Figure D.4 — Example of a flow chart of a fish fillet preparation line, including MAP, mincing and freezing process
165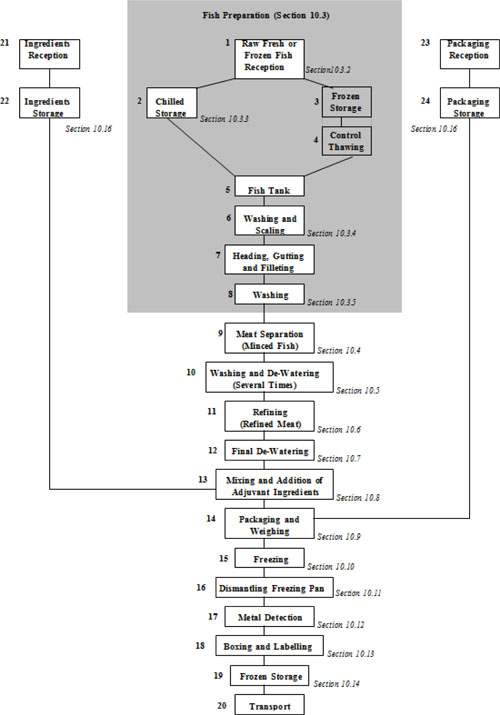
Figure D.5 — Example of a flow chart of a frozen surimi production process
166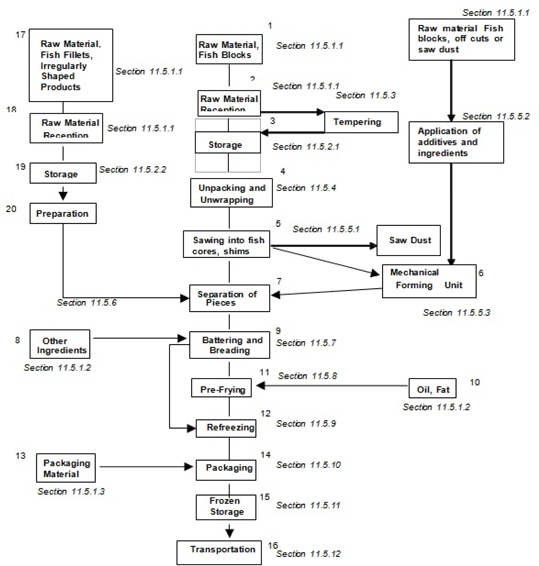
Figure D.6 — Example of a flow chart for the processing of coated fish products
NOTE The production and storage of batter for application to fish portions, fillets, etc., may involve either rehydration of a commercial batter mix or preparation from raw ingredients. During the preparation of this batter and its use, the potential hazard must be controlled.
167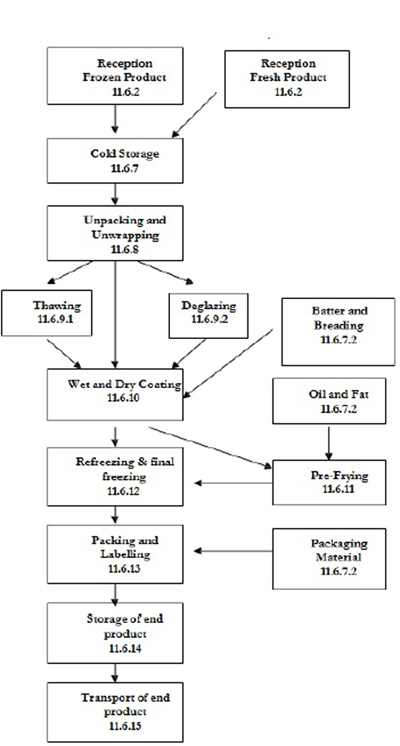
Figure D.7 — Example of a flow chart for coated molluscan shellfish processing
168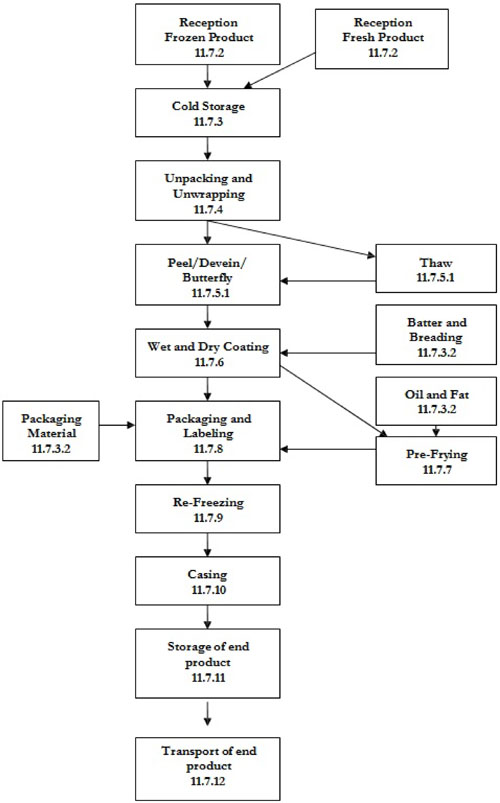
Figure D.8 — Example of a flow chart of a coated shrimp processing line
169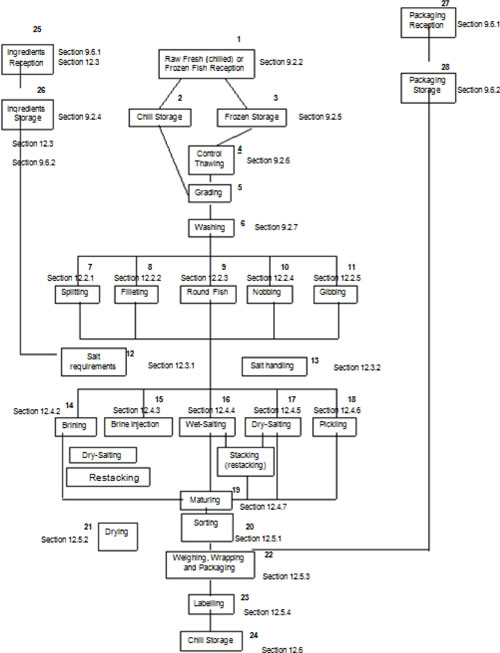
Figure D.9 — Example of a flow chart for salted and dried salted fish processing line
170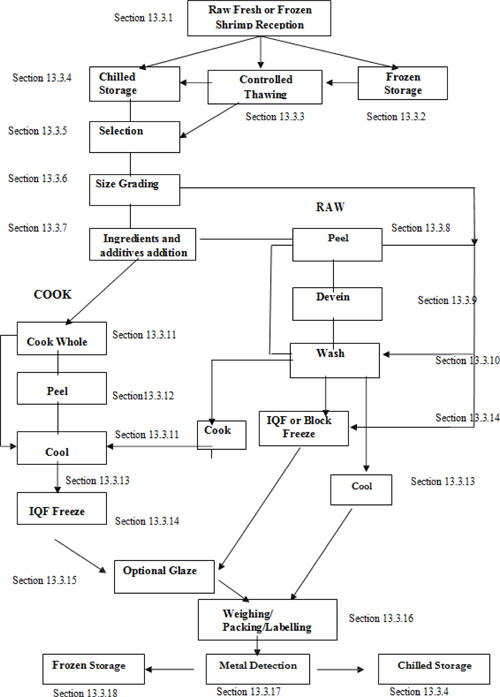
Figure D.10 — Example of a flow chart of a shrimp and prawn processing line
171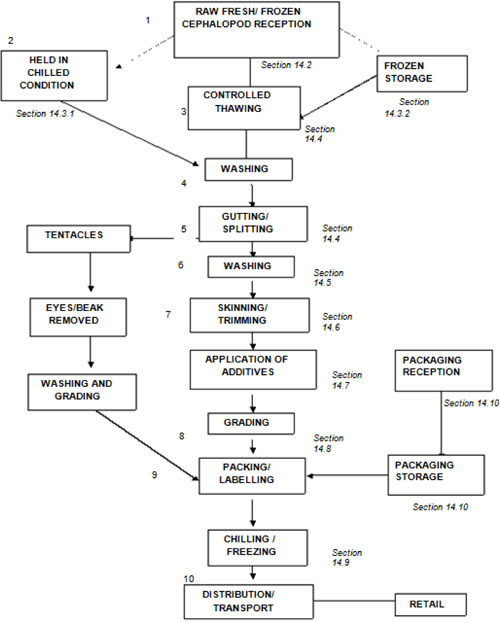
Figure D.11 — Example of a possible squid processing line
172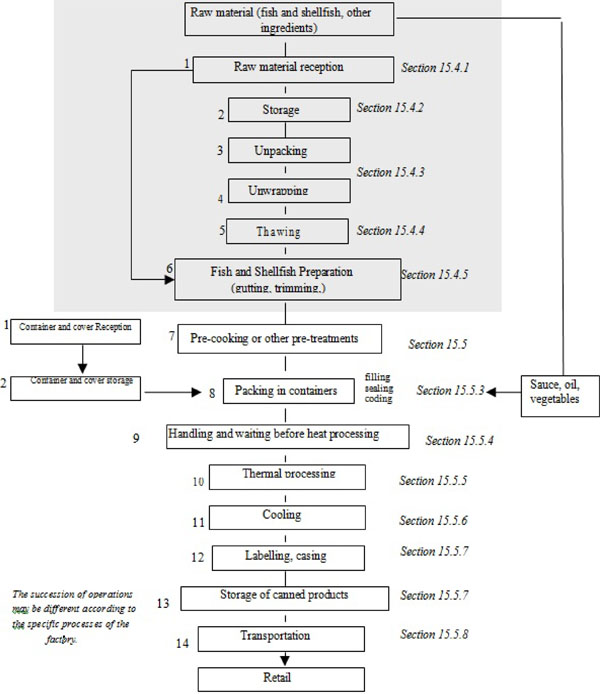
Figure D.12 — Example of a flow chart for the processing of canned fish and shellfish
173(normative)
Bivalve molluscs species like oysters, mussels, manilla and hard shell clams can survive for extended periods out of water and can be traded for human consumption as live animals. Other species like cockles can be traded live if carefully handled, but are normally processed. Species not adapted to dry conditions soon die out of water and are best handled as chilled products or processed.
NOTE When spawning (following “gonad ripening”) occurs, it becomes undesirable and in many instances impracticable to trade them as live animals. Stress can induce spawning.
The main hazard known for the production of bivalve molluscs is microbiological contamination of waters in which they grow, especially when the bivalve molluscs are intended to be eaten live or raw. Since molluscs are filter feeders they concentrate contaminants to a much higher concentration than the surrounding sea water. The contamination with bacteria and viruses in the growing area is therefore critical for the end product specification and determines the process requirements for further processing. Gastro-enteritis and other serious diseases such as hepatitis can occur as a result from agricultural run-off and or sewage contamination like enteric bacterial and or viral pathogens (Norovirus, viruses causing hepatitis) or from natural occurring bacterial pathogens (Vibrio spp.). Another hazard is formed by biotoxins. Biotoxins produced by some algae can cause various forms of serious poisoning like diarrhetic shellfish poisoning (DSP), paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), amnesic shellfish poisoning (ASP) or poisoning caused by Azaspiracid (AZP). Chemical substances, such as heavy metals, pesticides, organochlorides, petrochemical substances may also form a hazard in certain areas.
Control of hazards, identification and monitoring of growing areas is very important for bivalve molluscs safety. The identification, classification and monitoring of these areas should be carried out by the competent authorities in cooperation with fishermen and primary producers. E. coli/ or faecal coliforms or total coliforms may be used as an indicator for the possibility of faecal contamination. If biotoxins are found in the bivalve molluscs flesh in hazardous amounts the growing area must be closed for harvesting bivalve molluscs until toxicological investigation has made clear that the bivalve mollusc meat is free from hazardous amount of biotoxins. Harmful chemical substances should not be present in the edible part in such amounts that the calculated dietary intake exceeds the permissible daily intake.
Bivalve molluscs from waters subject to microbiological contamination, as determined by the authority having jurisdiction, can be made safe by relaying in a suitable area or a depuration process to reduce the level of bacteria if the process is continued long enough, or by processing to reduce or limit target organisms. Depuration is a short-term process commonly used to reduce low levels of bacterial contamination, but long term relaying is required if there is a greater risk of contamination.
Stress and excessive shocks of the bivalve molluscs must be avoided especially when the bivalve molluscs need to undergo relaying or depuration to be eaten live or raw. This is important because these bivalve molluscs should be able to function again during depuration, relaying or conditioning.
174(informative)
The best method of assessing the freshness or spoilage of fish is by sensory evaluation techniques. It is recommended that appropriate sensory evaluation criteria be used to evaluate the acceptability of fish and to eliminate fish showing loss of essential quality provisions of the appropriate CODEX standards. As an example, fresh white fish species are considered unacceptable when showing the following characteristics:
| Body part/Physical characteristic | Unacceptable characteristics |
| Skin / Slime | dull, gritty colours with yellow brown dotting slime |
| Eyes | Concave, opaque, sunken discoloured |
| Gills | grey – brown or bleached, slime opaque yellow, thick or clotting |
| Odour | flesh odour amines, ammonia, milky lactic, sulphide, faecal, putrid, rancid |
(informative)
These products specifications describe the optional defects for salted fish. The descriptions of optional defects will assist buyers and sellers in describing those defect provisions. These descriptions are optional and are in addition to the essential requirements prescribed in the appropriate CODEX product standards.
Reference is given to the CODEX Standard for Salted Fish and Dried Salted Fish of the Gadidae Family of Fishes (CODEX STAN 167-1989).
Products from the species as identified in Table G.1, all belong to the Gadidae family, should have been bled, gutted, beheaded and split so that approximately two thirds of the backbone is removed, washed and fully saturated with salt. Salted Fish used for production of Dried Salted Fish shall have reached 95 % salt saturation prior to drying.
| English name | Latin name |
|---|---|
| Blue ling | Molva dypterygia |
| Cod | Gadus morhua |
| Forkbeard | Phycis blennoides |
| Greenland cod | Gadus ogac |
| Haddock | Gadus aeglefinus / Melanogrammus aeglefinus |
| Ling | Molva molva |
| Pacific cod | Gadus macrocephalus |
| Polar cod | Boreogadus saida |
| Pollack | Pollachius pollachius |
| Saithe | Pollachius virens |
| Tusk | Brosme brosme |

The CARICOM Regional Organisation for Standards and Quality (CROSQ) was created as an Inter-Governmental Organisation by the signing of an agreement among fourteen Member States of the Caribbean Community (CARICOM). CROSQ is the regional centre for promoting efficiency and competitive production in goods and services, through the process of standardization and the verification of quality. It is the successor to the Caribbean Common Market Standards Council (CCMSC), and supports the CARICOM mandate in the expansion of intra-regional and extra-regional trade in goods and services.
CROSQ is mandated to represent the interest of the region in international and hemispheric standards work, to promote the harmonization of metrology systems and standards, and to increase the pace of development of regional standards for the sustainable production of goods and services in the CARICOM Single Market and Economy (CSME), and the enhancement of social and economic development.
CROSQ VISION:
The premier CARICOM organisation for the development and promotion of an Internationally Recognised Regional Quality Infrastructure; and for international and regional harmonized CARICOM Metrology, Standards, Inspection, Testing and Quality Infrastructure
CROSQ MISSION:
The promotion and development of standards and standards related activities to facilitate international competitiveness and the sustainable production of goods and services within the CARICOM Single Market and Economy (CSME) for the enhancement of social and economic development
177 178